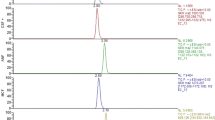
Abstract
A new, sensitive and fast method for the simultaneous determination of pyrazinamide, isoniazid, streptomycin, ethambutol, and rifampicin in human plasma was developed and validated. The method required only 100 μL of plasma and one step for sample preparation by protein precipitation. The drugs were separated by using a hydrophilic interaction liquid chromatography (HILIC) column. The mobile phase was methanol and water (0.1 % formic acid and 5 mM ammonium acetate, pH 3.0 ± 0.1) in a ratio of 65:35 (v/v), which was eluted at an isocratic flow rate of 0.5 mL/min. Tandem mass spectrometry was performed with a triple-quadrupole tandem mass spectrometer. By use of the HILIC column, the detection was free of ion-pair reagents in the mobile phase, with no significant matrix effects. The total run time was less than 2 min for each sample. The method was validated by evaluating its selectivity, sensitivity, linearity, accuracy, and precision according to US Food and Drug Administration guidelines. The lower limit of quantification was 4.0 ng/mL for pyrazinamide, isoniazid, and rifampicin, 0.5 ng/mL for ethambutol, and 10.0 ng/mL for streptomycin. The intraday precision and interday precision were less than 9 %, with the accuracy ranging between −9.3 and 7.3 %. The method was successfully applied to therapeutic drug monitoring of 33 patients with tuberculosis after administration of standard antituberculosis drugs. The method has been proved to meet the high-throughput requirements in therapeutic drug monitoring.

Scatter plots of 2-h plasma drug concentration of patients after receiving a standard medication. (The lower line and the upper line represent the low and high levels of the expected plasma concentrations of the antituberculosis drugs in tuberculosis patients. Open triangles female, age 0–20 years; closed triangles male, age 0–20 years; open diamonds female, age 21–40 years; closed diamonds male, age 21–40 years; open squares female, age 41–60 years; closed squares male, age 41–60; open circles female, age 61–80 years; closed circles male, age 61–80 years)





Similar content being viewed by others
References
WHO (2012) Global tuberculosis report 2012. Available via http://www.who.int/tb/publications/global_report/en/
WHO (2003) Treatment of tuberculosis: guideline for national programmes, 3rd edn. Word Health Organization, Geneva
Kayhan S, Akgüneş A (2011) Therapeutic monitoring of isoniazid, rifampicin, ethambutol and pyrazinamide serum levels in the treatment of active pulmonary tuberculosis and determinants of their serum concentrations. Afr J Pharm Pharmacol 5(17):2035–2041
Heysell SK, Moore JL, Keller SJ, Houpt ER (2010) Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 16(10):1546–1553
Peloquin CA (2002) Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62(15):2169–2183
Magis-Escurra C, van den Boogaard J, Ijdema D, Boeree M, Aarnoutse R (2012) Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm Pharmacol Ther 25(1):83–86
Taneja D, Kaur D (1990) Study on hepatotoxicity and other side-effects of antituberculosis drugs. J India Med Assoc 88:278–280
Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE (1998) Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 113(5):1178–1183
Tappero J, Bradford W, Agerton T, Hopewell P, Reingold A, Lockman S, Oyewo A, Talbot E, Kenyon T, Moeti T, Moffat H, Peloquin C (2005) Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–469
Ray J, Gardiner I, Marriott D (2003) Managing antituberculosis drug therapy by therapeutic drug monitoring of rifampicin and isoniazid. Intern Med J 33(5–6):229–234
Allanson AL, Cotton MM, Tettey JNA, Boyter AC (2007) Determination of rifampicin in human plasma and blood spots by high performance liquid chromatography with UV detection: a potential method for therapeutic drug monitoring. J Pharm Biomed Anal 44(4):963–969
Guermouche S, Guermouche MH (2004) Solid-phase extraction and HPTLC determination of isoniazid and acetylisoniazid in serum. Comparison with HPLC. J Chromatogr Sci 42(5):250–253
Breda M, Marrari P, Pianezzola E, Strolin Benedetti M (1996) Determination of ethambutol in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 729(1–2):301–307
Zhou Z, Chen L, Liu P, Shen M, Zou F (2010) Simultaneous determination of isoniazid, pyrazinamide, rifampicin and acetylisoniazid in human plasma by high-performance liquid chromatography. Anal Sci 26(11):1133–1138
Moussa LA, Khassouani CE, Soulaymani R, Jana M, Cassanas G, Alric R, Hüe B (2002) Therapeutic isoniazid monitoring using a simple high-performance liquid chromatographic method with ultraviolet detection. J Chromatogr B 766(1):181–187
Khuhawar MY, Rind FMA (2002) Liquid chromatographic determination of isoniazid, pyrazinamide and rifampicin from pharmaceutical preparations and blood. J Chromatogr B 766(2):357–363
Sarri AK, Megoulas NC, Koupparis MA (2006) Development of a novel method based on liquid chromatography-evaporative light scattering detection for the direct determination of streptomycin and dihydrostreptomycin in raw materials, pharmaceutical formulations, culture media and plasma. J Chromatogr A 1122(1–2):275–278
Khuhawar MY, Zardari LA (2006) Capillary gas chromatographic determination of isoniazid in pharmaceutical preparations and blood by precolumn derivatization with trifluoroacetylacetone. J Food Drug Anal 14(4):323–328
Malone RS, Fish DN, Spiegel DM, Childs JM, Peloquin CA (1999) The effect of hemodialysis on isoniazid, rifampin, pyrazinamide, and ethambutol. Am J Respir Crit Care Med 159(5):1580–1584
Huang L, Marzan F, Jayewardene AL, Lizak PS, Li X, Aweeka FT (2009) Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for determination of isoniazid in human plasma. J Chromatogr B 877(3):285–290
Wang A, Zhang W, Sun J, Liu JF, Sang YS, Gao S, Hee ZG (2007) HPLC-MS analysis of isoniazid in dog plasma. Chromatographia 66:741–745
Granados O, Meza G (2007) A direct HPLC method to estimate streptomycin and its putative ototoxic derivative, streptidine, in blood serum: application to streptomycin-treated humans. J Pharm Biomed Anal 43(2):625–630
Chen XY, Song B, Jiang HJ, Yu K, Zhong DF (2005) A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of isoniazid and ethambutol in human plasma. Rapid Commun Mass Spectrom 19(18):2591–2596
Gong Z, Basir Y, Chu D, McCort-Tipton M (2009) A rapid and robust liquid chromatography/tandem mass spectrometry method for simultaneous analysis of anti-tuberculosis drugs—ethambutol and pyrazinamide in human plasma. J Chromatogr B 877(16–17):1698–1704
Song SH, Jun SH, Park KU, Yoon Y, Lee JH, Kim JQ, Song J (2007) Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21(7):1331–1338
Minikis RM, Dolan JW (2003) Extracolumn effects. LCGC N Am 21(11):1050–1054
US Department of Health and Human Services, Food and Drug Administration (2001) Guidance for industry, bioanalytical method validation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
European Medicines Agency (2011) Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009. http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500109686&murl=menus/document_library/document_library.jsp&mid=WC0b01ac058009a3dc
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75(13):3019–3030
World Anti-Doping Agency (2010) WADA technical document TD2010IDCR. World Anti-Doping Agency, Montreal
Fang PF, Cai HL, Li HD, Zhu RH, Tan QY, Gao W, Xu P, Liu YP, Zhang WY, Chen YC, Zhang F (2010) Simultaneous determination of isoniazid, rifampicin, levofloxacin in mouse tissues and plasma by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B 878(24):2286–2291
Gremilogianni AM, Megoulas NC, Koupparis MA (2010) Hydrophilic interaction vs ion pair liquid chromatography for the determination of streptomycin and dihydrostreptomycin residues in milk based on mass spectrometric detection. J Chromatogr A 1217(43):6646–6651
Garcı́a MC, Hogenboom AC, Zappey H, Irth H (2002) Effect of the mobile phase composition on the separation and detection of intact proteins by reversed-phase liquid chromatography–electrospray mass spectrometry. J Chromatogr A 957(2):187–199
Hemström P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29(12):1784–1821
Jandera P (2011) Stationary and mobile phases in hydrophilic interaction chromatography: a review. Anal Chim Acta 692(1–2):1–25
Nguyen HP, Schug KA (2008) The advantages of ESI-MS detection in conjunction with HILIC mode separations: fundamentals and applications. J Sep Sci 31(9):1465–1480
Zhu M, Burman WJ, Jaresko GS, Berning SE, Jelliffe RW, Peloquin CA (2001) Population pharmacokinetics of intravenous and intramuscular streptomycin in patients with tuberculosis. Pharmacotherapy 21(9):1037–1045
Magis-Escurra C, van den Boogaard J, Ijdema D, Boeree M, Aarnoutse R (2012) Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm Pharmacol Ther 25(1):83–86
Fahimi F, Kobarfard F, Tabarsi P, Hemmati S, Salamzadeh J, Baniasadi S (2011) Isoniazid blood levels in patients with pulmonary tuberculosis at a tuberculosis referral center. Chemotherapy 57(1):7–11
Holland DP, Hamilton CD, Weintrob AC, Engemann JJ, Fortenberry ER, Peloquin CA, Stout JE (2009) Therapeutic drug monitoring of antimycobacterial drugs in patients with both tuberculosis and advanced human immunodeficiency virus infection. Pharmacotherapy 29(5):503–510
Notari S, Mancone C, Sergi M, Gullotta F, Bevilacqua N, Tempestilli M, Urso R, Lauria FN, Pucillo LP, Tripodi M, Ascenzi P (2010) Determination of antituberculosis drug concentration in human plasma by MALDI-TOF/TOF. IUBMB Life 62(5):387–393
Acknowledgments
This work was financially supported by grants from the National Basic Research Program of China (no. 2012CB518200 and no. 30971193) and the School of Public Health and Tropical Medicine, Southern Medical University, China (no. GW201118).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhifeng Zhou and Xianbo Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, Z., Wu, X., Wei, Q. et al. Development and validation of a hydrophilic interaction liquid chromatography–tandem mass spectrometry method for the simultaneous determination of five first-line antituberculosis drugs in plasma. Anal Bioanal Chem 405, 6323–6335 (2013). https://doi.org/10.1007/s00216-013-7049-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7049-0