Abstract
Rationale
To enhance the effectiveness of antipsychotics in first-episode psychosis is crucial in order to achieve the most favourable prognosis. Difference in effectiveness between antipsychotics is still under debate.
Objective
The purpose of this study is to determine the long-term (3-year) effectiveness and efficacy of haloperidol, risperidone and olanzapine in first-episode schizophrenia-spectrum disorders.
Method
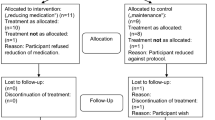
This is a prospective, randomized, open-label study. Data for the present investigation were obtained from a large epidemiologic and 3-year longitudinal intervention programme of first-episode psychosis. One hundred seventy-four patients were randomly assigned to haloperidol (N = 56), olanzapine (N = 55), or risperidone (N = 63) and followed up for 3 years. The primary effectiveness measure was all-cause of treatment discontinuation. In addition, an analysis based on per-protocol populations was conducted in the analysis for clinical efficacy.
Results
The treatment discontinuation rate for any cause differed significantly between treatment groups (χ 2 = 10.752; p = 0.005), with a higher rate in haloperidol than in risperidone and olanzapine. The difference in the discontinuation rate between risperidone and olanzapine showed a tendency towards significance (χ 2 = 3.022; p = 0.082). There was a significant difference in the mean time to all-cause discontinuation between groups (log-rank χ 2 = 12.657;df = 2; p = 0.002). There were no significant advantages to any of the three treatments in reducing the psychopathology severity.
Conclusions
After 3 years of treatment, a lower effectiveness was observed in haloperidol compared to second-generation antipsychotics (SGAs). The use of SGAs for the treatment of early phases of nonaffective psychosis may enhance the effectiveness of antipsychotics.


Similar content being viewed by others
References
Addington D, Addington J, Malika-tyndale E (1993) Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry (Suppl) 22:39–44
Andreasen NC (1983) Scale for the Assessment of Negative symptoms (SANS). University of Iowa, Iowa City
Andreasen NC (1984) Scale for the Assessment of Positive symptoms (SAPS). University of Iowa, Iowa City
Barnes TR (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676
Crespo-Facorro B, Perez-Iglesias R, Ramirez-Bonilla ML et al (2006) A practical clinical trial comparing haloperidol, risperidone and olanzapine for the acute treatment of first-episode nonaffective psychosis. J Clin Psychiatry 67:1511–1521
Crespo-Facorro B, Perez-Iglesias R, Mata I et al (2011) Effectiveness of haloperidol, risperidone and olanzapine in the treatment of first-episode non-affective psychosis: results of a randomized, flexible-dose, open-label 1-year follow-up comparison. J Psychopharmacol 25:744–754
First MB, Spitzer RL, Gibbon M, William JB (2001) Structured Clinical Interview for DSM-IV-TR Axis I Disorders—non-patient edition. Biometrics Research Department, New York
Gaebel W, Riesbeck M, Wölwer W et al (2007) Maintenance treatment with risperidone or low-dose haloperidol in first-episode schizophrenia: 1-year results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry 68:1763–1774
Green AI, Lieberman JA, Hamer RM et al (2006) Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res 86:234–243
Guy W (1976) ECDEU Assessment manual for psychopharmacology. US Department of Health, Education, and Welfare publication ADM 76338. National Institute of Mental Health, Washington, DC, pp 217–222
Haro JM, Novick D, Suarez D et al (2009) Antipsychotic treatment discontinuation in previously untreated patients with schizophrenia: 36-month results from the SOHO study. J Psychiatr Res 43:265–273
Janca A, Kastrup M, Katschnig H et al (1996) The World Health Organization Short Disability Assessment Schedule (WHO DAS-S): a tool for the assessment of difficulties in selected areas of functioning of patients with mental disorders. Soc Psychiatry Psychiatr Epidemiol 31:349–354
Kahn RS, Fleischhacker WW, Boter H et al (2008) Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097
Kelly DL, Conley RR, Carpenter WT (2005) First-episode schizophrenia. A focus on pharmacological treatment and safety considerations. Drugs 65:1113–1138
Leucht S, Arbter D, Engel RR et al (2009a) How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14:429–447
Leucht S, Corves C, Arbter D et al (2009b) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31–41
Lieberman JA (1996) Atypical antipsychotics drugs as a first-line treatment of schizophrenia: rationale and hypothesis. J Clin Psychiatry (Suppl) 11:68–71
Lieberman JA, Tollefson G, Tohen M et al (2003) Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry 160:1396–1404
Lingjarde O, Ahlfors UG, Bech P et al (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and cross sectional study of side effects in antipsychotic-treated patients. Acta Psychiatr Scand (Suppl) 334:1–100
March JS, Silva SG, Compton S et al (2005) The case for practical clinical trials in psychiatry. Am J Psychiatry 162:836–846
McEvoy JP, Lieberman JA, Perkins DO et al (2007) Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164:1050–1060
Miller IW, Bishop S, Norman WH et al (1985) The modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res 14:131–142
Overall JE, Gorham DR (1962) The Brief Psychiatric Rating Scale. Psychol Rep 10:799–812
Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M et al (2008) Epidemiological factors associated with treated incidence of first-episode non-affective psychosis in Cantabria: insights from the PAFIP. Early Interv Psychiatry 2:178–187
Schooler N, Rabinowitz J, Davidson M et al (2005) Risperidone and haloperidol in first-episode psychosis: a long-term randomised trial. Am J Psychiatry 162:947–953
Simpson GM, Angus JWS (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand (Suppl) 212:11–19
Wyatt RJ (1991) Neuroleptics and the natural course of schizophrenia. Schizophr Bull 17:325–351
Young RC, Biggs JT, Ziegler VE et al (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–433
Acknowledgements
We wish to thank the PAFIP researchers who helped with data collection and specially acknowledge Mrs. Gema Pardo for data collection. Finally, we would also like to thank the participants and their families for enrolling in this study.
Conflict of interest disclosures
Prof. Vazquez-Barquero and Prof. Crespo-Facorro have received unrestricted research funding from AstraZeneca, Pfizer, Bristol-Myers Squibb and Johnson & Johnson that was deposited into research accounts at the University of Cantabria. Prof. Vazquez-Barquero has received honoraria for his participation as a speaker at educational events from Johnson & Johnson. Prof. Crespo-Facorro has received honoraria for his participation as a speaker at educational events from Pfizer, Bristol-Myers Squibb and Johnson & Johnson and consultant fees from Pfizer. Dr. Mata has received honoraria for his participation as a speaker at educational events from Johnson & Johnson. Dr. Perez-Iglesias has received support to attend conferences from Lilly. Drs. Pelayo-Teran and Valdizan, Mrs. Martinez and Mr. Ortiz report no additional financial or other relationship relevant to the subject of this article.
Funding
The present study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain under the following grant support: Instituto de Salud Carlos III PI020499, PI050427, PI060507, Plan Nacional de Drogas Research Grant 2005-Orden sco/3246/2004, SENY Fundació Research Grant CI 2005–0308007 and Fundación Marqués de Valdecilla API07/011.
No pharmaceutical company supplied any financial support towards it. The study, designed and directed by B C-F and JL V-B, conformed to international standards for research ethics and was approved by the local institutional review board.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 106 kb)
Rights and permissions
About this article
Cite this article
Crespo-Facorro, B., Pérez-Iglesias, R., Mata, I. et al. Long-term (3-year) effectiveness of haloperidol, risperidone and olanzapine: results of a randomized, flexible-dose, open-label comparison in first-episode nonaffective psychosis. Psychopharmacology 219, 225–233 (2012). https://doi.org/10.1007/s00213-011-2392-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2392-3