Abstract
Aims/hypothesis
Anti-CD3 monoclonal antibodies remain the most promising immune therapy for reversing recent-onset type 1 diabetes. However, current clinical trials have revealed their major drawback, namely the narrow therapeutic window in which low doses are ineffective and higher doses that preserve functional beta cell mass cause side effects. Strategies that sidestep these limitations while preserving or improving anti-CD3’s therapeutic efficiency are essential. We hypothesised that combining a potent vitamin D3 analogue (TX527), ciclosporin A (CsA) and anti-CD3 would act to lower the dose while maintaining or even boosting therapeutic efficacy to counteract autoimmune destruction of transplanted islets.
Methods
This study involved the use of syngeneic islet transplantation, immunofluorescence microscopy, immune phenotyping by flow cytometry, RT-PCR analysis, and in vitro and in vivo suppression assays.
Results
Combination therapy with TX527, CsA and anti-CD3 was well tolerated on the basis of weight, bone and calcium variables. Remarkably, combining all three agents at sub-therapeutic doses greatly reduced recurrent autoimmune responses to a grafted islet mass (mean ± SEM: 79.5 ± 18.6 days; p < 0.01), by far exceeding the therapeutic efficacy of monotherapy (24.8 ± 7.3 days for anti-CD3) and dual therapy (25.5 ± 12.4 days for anti-CD3+CsA). Combination therapy surpassed anti-CD3 monotherapy in reducing islet infiltration by effector/memory phenotype CD8+ T cells, as well as by reducing proinflammatory cytokine responses and increasing the frequency of T regulatory cells that were functional in vitro and in vivo, and acted in a cytotoxic T lymphocyte antigen 4-dependent manner.
Conclusions/interpretation
Combining the immunomodulatory actions of anti-CD3 mAb with CsA and the vitamin D3 analogue, TX527, delivers therapeutic efficacy in an islet-transplanted NOD mouse model of diabetes.
Similar content being viewed by others
Introduction
In recent years, several strategies have been put forward to prevent or curb the autoimmune response in type 1 diabetes, a disease of the pancreas in which autoreactive T cells attack the beta cells in the islets of Langerhans [1, 2]. The first results with anti-CD3 monoclonal antibodies (mAbs) were very promising, showing that a short treatment course with these T cell targeting mAbs could successfully reverse recent-onset type 1 diabetes and induce long-term disease remission in a model of diabetes-prone NOD mice [3, 4]. Also, in human trials, anti-CD3 mAb therapy has shown great promise, especially in patients who still have a large functional beta cell mass when therapy is initiated, resulting in reduced requirements for exogenous insulin in the months after therapy [5–7].
The major drawback of anti-CD3 mAb therapy is its narrow therapeutic window formed on the high-dose side by clinical adverse events, such as a transient cytokine release syndrome causing flu-like symptoms and temporal re-activation of Epstein–Barr virus infections [5, 7, 8], and on the low-dose side by the loss of therapeutic efficacy [9]. Such setbacks have driven the search for alternative strategies, not only to overcome the limitations but also to improve the therapeutic efficacy of anti-CD3 monotherapy.
1α,25-Dihydroxyvitamin D3, the active metabolite of vitamin D3, and its synthetic analogues with decreased hypercalcaemia liability are potent immunomodulators which target multiple central players of the autoimmune processes in type 1 diabetes. Vitamin D3 exerts its immunomodulatory activities at supraphysiological doses [10], hence less calcaemic analogues are preferred. As such, vitamin D3 analogues induce T regulatory cells (Tregs) in vitro and in vivo [11–13] and skew antigen-presenting cells (APCs), such as dendritic cells and macrophages, towards a tolerogenic profile [14–16]. As shown by our group, these properties culminate in the prevention of insulitis and type 1 diabetes in NOD mice [17–19]. Late administration of vitamin D3 analogues cannot halt ongoing autoimmunity in NOD mice, but co-administration of other immunomodulating agents greatly enhances their disease-modifying abilities [20–22]. In particular, combinations of vitamin D3 analogues and calcineurin inhibitors such as ciclosporin A (CsA) are highly effective in preventing spontaneous diabetes [21] or the autoimmune destruction of syngeneic islet transplants in NOD mice [22]. The use of low doses of CsA almost doubled the frequency of circulating Forkhead box P3 (FOXP3)+ CD4+ Tregs in patients with atopic dermatitis [23], indicating that this compound does not counteract in vivo Treg induction per se, as indicated in other studies [24].
The effectiveness of immunotherapies for autoimmune diabetes is often tested in recent-onset diabetes in a NOD mouse model [25]. In such a setting, the success of the immunotherapy not only depends on whether the autoimmune response is actually blocked or deviated, but also on the functional beta cell mass present, whether residual, regenerated or revived. In the recurrence model used here, syngeneic islets are transplanted to restore normoglycaemia in diabetic NOD mice, thus eliminating the variable factor of residual beta cell mass. Typically after syngeneic islet transplantation, the autoimmune response active in diabetic recipients rapidly destroys the transplanted beta cell mass unless a successful immunotherapeutic intervention is administered. The advantage is that this model is independent of remaining beta cell mass and links the immunological efficacy directly to the clinical outcome. In this model, we found that combination immunotherapy with sub-therapeutic doses of anti-CD3 mAb, CsA and the bioactive vitamin D3 analogue, TX527, delays diabetes recurrence after syngeneic islet transplantation.
Methods
Diabetes follow-up in NOD mice
NOD mice, originally obtained from Professor C.Y. Wu (Beijing, China), have been bred in our animal facility since 1989 under semi-barrier conditions. Diabetes incidence in the colony was 75% in female and 45% in male mice at the time of the study. Diabetes was determined as two consecutive days of hyperglycaemia (>11.1 mmol/l). Severely diabetic male and female mice (mean initial blood glucose concentrations of 25.5 ± 5.4 mmol/l) were selected as recipients for grafting. All animal breeding and experimental protocols were approved by the ethics committee of the Katholieke Universiteit Leuven (KU Leuven).
Treatment substances
Whole anti-CD3 mAb (145-2C11, hamster IgG) was kindly provided by Professor L. Chatenoud (Inserm, Paris, France). The vitamin D3 analogue, TX527 [19-nor-14,20-bisepi-23-yne-1,25(OH)2D3], was synthesised by M. Vandewalle and P. de Clercq (University of Ghent, Belgium) and obtained from Théramex S.A. (Monaco). CsA was obtained from Novartis (Basel, Switzerland). For in vivo administration, TX527 and CsA were dissolved in peanut oil; anti-CD3 mAb was diluted in 0.9% NaCl.
Islet isolation and transplantation and treatment regimen
Islets were prepared from 14–21-day-old insulitis-free NOD donor mice and transplanted under the kidney capsule of diabetic NOD mice, as previously described [20]. Transplanted mice were either left untreated or treated with sub-therapeutic doses of anti-CD3 mAb (2.5 μg/day, i.v., days 0–4), TX527 (10 μg/kg every 2 days, i.p., day 1 until day 60) or CsA (5 mg/kg per day, by mouth, day 1 until day 60) or a combination of these three agents (combination therapy, CT). In electronic supplementary material (ESM) Fig. 1, the transplanted mice received a combination of TX527 (5 μg/kg per day, i.p., day 1 until day 30) and CsA (7.5 mg/kg per day, by mouth, day 1 until day 20). The anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) mAb (clone UC10-4F10) dose regimen was as follows: 250 μg i.p. on days 0 and 2, then 100 μg on days 7, 9, 11, 14 and 19. In ESM Fig. 3, diabetic mice did not receive islet transplants and were treated with anti-CD3 mAb only.
Histology and immunohistochemistry
On days 10 or 21 after islet transplantation, graft-bearing kidneys were removed and fixed in 4% formaldehyde followed by paraffin embedding, or embedded and frozen in Tissue-Tek OCT compound (Sakura, Alphen aan den Rijn, the Netherlands) for cryosectioning. Morphology of the grafts was assessed on serial sections (5–7 μm) stained with haematoxylin and eosin (H&E). For immunohistochemical analysis, frozen sections were stained as described by Sutherland et al [26] and mounted in Vectamount Aqua (Vector Labs, Burlingame, CA, USA). Images were acquired with a ×20 objective on the Zeiss AxioPlan2 imaging system (Zeiss, Zaventem, Belgium) using AxioVision imaging software (Zeiss).
In vitro assays
Splenocytes, isolated from 21-day transplanted mice, were cultured in quadruplicate in round-bottom 96-well plates (2 × 105 cells in 200 μl), and either left unstimulated or stimulated with soluble anti-CD3 (145-2C11, 1 μg/ml; eBioscience, San Diego, CA, USA) or concanavalin A (ConA; 6.25 μg/ml; Sigma-Aldrich, St Louis, MO, USA) for 72 h. During the last 16 h of cell culture, cells were pulsed with [3H]thymidine (1 μCi/well), after which 3H incorporation was determined by liquid-scintillation counting. For cytokine analysis, supernatant fractions of ConA-stimulated splenocytes were collected after 56 h and analysed using the Mouse Th1/Th2/Th17/Th22 13plex FlowCytomix Multiplex kit (Bender Medsystems, eBioscience, San Diego, CA, USA), as specified by the manufacturer. Data were acquired on a Gallios flow cytometer (Beckman Coulter, Indianapolis, IN, USA) and analysed with FlowCytomix Pro 2.3 (Bender Medsystems).
Real-time RT-PCR for cytokines
mRNA quantities of indicated cytokines were quantified as previously determined [27]. The data were analysed using the comparative Ct method, in which the amount of target, normalised to an endogenous reference gene (normalisation gene) and relative to a calibrator (e.g. an untreated control sample), is given by \( {2^{{ - \Delta \Delta {{\text{C}}_{\text{t}}}}}} \), where ΔCt = Cttarget gene − Ctnormalisation gene and ΔΔCt = ΔCtsample − ΔCtcalibrator. All samples were normalised to the average of β-actin, hydroxymethylbilane synthase (HMBS) and ribosomal protein L27 (RPL27). Background quantities of each target gene were calculated from the non-grafted kidney.
Flow cytometry
Blood and the indicated organs were harvested, processed and then incubated with fluorochrome-labelled mAbs against Thy1.2, T cell receptor (TCR)-β (BD Biosciences, San Jose, CA, USA), CD3, CD4, CD8, FOXP3 (eBioscience), CD44 (Biolegend, San Diego, CA, USA) or matching isotype controls for 20 min on ice. After being washed, samples were acquired on a FACS Canto (BD Biosciences) or Gallios flow cytometer and analysed using the FACSDiva (BD Biosciences) or Kaluza software (Beckman Coulter), respectively.
In vitro and in vivo suppression assay
In vitro responder T cells were negatively isolated as CD4+CD25− from spleen and pancreatic lymph nodes (PLN) of non-diabetic NOD mice (Dynabeads; Invitrogen, Carlsbad, CA, USA) and labelled with eFluor 670 Proliferation Dye (eBioscience). Splenocytes from NOD Scid (also known as Prkdc) γc−/− mice served as accessory cells. CD4+CD25+ T cells were isolated by a CD4+-enrichment step followed by a CD25+-selection step using biotinylated anti-CD25 and anti-biotin microbeads (Miltenyi, Bergisch Gladbach, Germany). Suppression assays were performed in round-bottom 96-well plates containing 5 × 104 responder T cells, 1 × 105 accessory cells, soluble anti-CD3 mAb (145-2C11, 1 μg/ml) and CD4+CD25+ T cells isolated from untreated or CT-treated transplant-recipient NOD mice as putative suppressor cells at indicated suppressor/responder ratios. Where indicated, blocking antibodies, hamster anti-mouse CTLA-4 mAb (UC10-4F10; kindly provided by Louis Boon, Bioceros BV, Utrecht, the Netherlands) or anti-IL-10 (JES5-2A5; BioXell, West Lebanon, NH, USA), were added at 10 μg/ml. After 72 h, responder T cell proliferation was determined by flow cytometric analysis of eFluor 670 Proliferation Dye dilution. In vivo suppressive capacity was assessed by glycaemia monitoring of 6–8-week-old NOD Scid mice upon transfer of 9 × 106 CD25-depleted diabetic NOD splenocytes with or without 1 × 106 CD4+CD25+ cells from untreated, anti-CD3-treated or CT-treated transplant recipients.
Calcium and bone variables
On day 10 after transplantation, mice were killed to collect blood serum by heart puncture and to isolate the femurs. Calcium in serum and femur was determined by a microcolorimetric assay (Sigma) [19]. Serum osteocalcin concentrations were determined with an in-house radioimmunoassay using mouse osteocalcin as a standard and polyclonal guinea pig anti-mouse osteocalcin serum. The sensitivity of this assay is 0.02 nmol/l. The calcium content of the femur was determined as described previously [15].
Statistical analysis
Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA). Statistical significance was calculated by the logrank test, ANOVA or Mann–Whitney test, as appropriate and indicated. For all tests, data were considered significantly different at p < 0.05.
Results
Combination therapy with sub-therapeutic doses of anti-CD3, TX527 and CsA prevents recurrence of autoimmune islet destruction
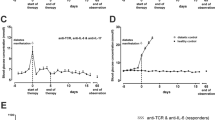
Restoration of functional beta cell mass in recent-onset diabetic NOD mice was achieved by transplantation of syngeneic islets, restoring normoglycaemia within 2 days in all animals (Fig. 1a, b). Autoimmune diabetes recurred in all animals, rendering them diabetic again within a maximum of 15 days (Fig. 1a, b; ESM Table 1). Sub-therapeutic doses of TX527 and CsA, alone or combined, did not significantly prolong the diabetes-free period (Fig. 1a), although the combination at a higher dose produced a modest delay of diabetes recurrence (ESM Fig. 1). On the other hand, low-dose anti-CD3 monotherapy significantly delayed diabetes recurrence (ESM Table 1, Fig. 1a). Dual treatment with sub-therapeutic doses of anti-CD3 plus either TX527 or CsA did not improve the effect of anti-CD3 alone, except for preventing primary graft non-function (Fig. 1b). Strikingly, islet-transplanted mice receiving all three drugs (CT) remained diabetes-free for 79.5 ± 18.6 days (mean ± SEM) (Fig. 1a). Protection by this CT was well tolerated, without bone decalcification (ESM Table 2) or weight loss (data not shown). In addition, no significant increase in bone turnover was observed, as evidenced by serum osteocalcin concentrations. Serum calcium concentrations were marginally increased after 10 days of treatment, but normalised once treatment ended (>60 days), indicating that the CT induces limited and transient calcaemic effects (ESM Table 2).
Prolonged graft acceptance on CT with low-dose anti-CD3, CsA and TX527. (a, b) Diabetic NOD mice received 500 syngeneic islets from 14–21-day-old NOD pups together with monotherapy (a) or CT (b). (a) Circles, untreated; squares, anti-CD3; diamonds, TX527; upside-down triangles, CsA. (b) Circles, untreated; squares, anti-CD3+TX527; diamonds, anti-CD3+CsA; upside-down triangles, TX527+CsA; triangles, anti-CD3+TX527+CsA (CT). Shown is the percentage of normoglycaemic mice per group as a measure of graft functionality. Also see ESM Table 1. Statistical significance was calculated by logrank test; ***p < 0.001. (c, d) H&E staining of cryosections of graft/kidney harvested from mice on day 10 (c) or 21 (d) after transplantation of syngeneic islets. (e, f) Immunohistochemistry staining for insulin (blue) and CD4 (red, e) or CD8 (red, f) on cryosections of graft/kidney harvested from mice on day 21 after transplantation of syngeneic islets. CTR, control; aCD3, anti-CD3
To assess whether the immunotherapy interfered with the immune infiltrate attacking the transplanted islets, histology of the transplanted islets was performed. Without immunotherapy, the islet grafts were already severely infiltrated and mostly destroyed after 10 days. At that time point, both anti-CD3 alone and CT preserved islet architecture and limited the immune infiltration of the transplanted islets (Fig. 1c). On day 21, however, anti-CD3 alone no longer dammed islet graft infiltration of either CD4+ or CD8+ T cells (Fig. 1d). In contrast, islet grafts of CT-treated mice were in general better preserved and less infiltrated by CD4+ and, especially, CD8+ T cells (Fig. 1e, f).
We next examined the intragraft amounts of the T cell-related cytokines on days 10 and 21 after transplantation. On day 10, CT and anti-CD3 both reduced the amounts of mRNA for Ifng, Tnfa, Il10 and Foxp3 (ESM Fig. 2). On day 21, Ifng and Tnfa were still reduced, but Foxp3 had increased in CT-treated mice (ESM Fig. 2).
Taken together, CT with anti-CD3, CsA and TX527 reduced the autoimmune attack on transplanted islets and prolonged the diabetes-free phase.
CT temporarily reduces the frequency of CD4+ T cells
Immunotherapies preferably achieve immune modulation without immune depletion. We first assessed the impact of low-dose anti-CD3 treatment on T cell depletion in a time kinetic and found that, in line with the immune phenotype described for high-dose anti-CD3 [28], low-dose anti-CD3 transiently depleted T cells and caused antigenic modulation in spleen and blood (data not shown). In blood, FOXP3+ Tregs were transiently increased when measured as a relative fraction of CD4+ T cells (ESM Fig. 3a), but not when measured as total percentage of cells in the blood (ESM Fig. 3b).
We studied the immune phenotype of CT-treated islet recipients in further detail on days 10 and 21 after transplantation, i.e. when anti-CD3-induced T cell depletion was still apparent or had recovered, respectively. On day 10, anti-CD3 alone and CT decreased the CD4+ T cell frequency in the spleen and the kidney draining lymph nodes (KDLNs) of islet recipients (Fig. 2a, d). In the KDLNs, this decrease was accompanied by a rise in CD8+ T cell frequency (Fig. 2e), but the CD4/CD8 ratio was nevertheless reduced in both spleen and KDLNs (Fig. 2c, f). Even though bulk CD4+ and CD8+ T cells normalised by day 21 (Fig. 2a–f), the distribution of CD4+ and CD8+ T cell subsets remained altered (Fig. 3a, b). As such, the anti-CD3 and CT increased antigen-experienced (CD44high) T cell fractions in CD4+ and CD8+ T cells in the spleen (Fig. 3b, f), but not in blood (Fig. 3a, e), and in CD8+ T cells, but not in CD4+ T cells, in the KDLNs (Fig. 3c, g). Importantly, CT treatment also reduced activated T cells locally, as evidenced by the lower proportion of CD44highCD4+ and CD44highCD8+ T cells in the islet grafts (Fig. 3d, h).
Reduced CD4+ T cells in the KDLNs on CT. Spleen (a–c) and KDLNs (d–f) were harvested on days 10 or 21 after syngeneic islet transplantation. The frequencies of CD4+ and CD8+ T cell subsets were measured by flow cytometry and displayed as percentage CD4+ (a, d) or CD8+ (b, e) of lymphocytes, or as ratio of CD4+ to CD8+ (c, f) per individual mouse. Symbols, line and error bars reflect individual values, mean and SEM, respectively. Statistical significance was calculated by Mann–Whitney test; ns, not significant; *p < 0.05. CTR, control; aCD3, anti-CD3
CT reduces activated CD4+ and CD8+ T cells in the islet graft. On day 21 after islet transplantation, blood (a, e), spleen (b, f), KDLNs (c, g) and islet grafts (d, h) were isolated from untreated (CTR), anti-CD3 (aCD3)- or CT-treated groups, as indicated. The frequencies of CD44high, CD4+ or CD8+ T cells were determined and plotted as percentage of CD4+ (a–d) or CD8+ (e–h) T cells. Bar graphs represent the mean with SEM. Statistical significance was calculated by Mann–Whitney test; *p < 0.05; **p < 0.01
We next assessed the potential of T cells to traffic to sites of inflammation. CT did not differ from anti-CD3 monotherapy in the upregulation of CXCR3 (CXC chemokine receptor 3, CD183) and CCR5 (C-C chemokine receptor 5, CD195) on CD4+ T cells, or in the transient upregulation of CXCR3 and downregulation of CCR5 on CD8+ T cells (ESM Fig. 4a, b). CXCR6 (CD186), although only expressed by a small fraction of the T cells, trended towards a higher positive fraction of CD4+ T cells by CT and a lower fraction of CD8+ T cells, compared with anti-CD3 monotherapy (ESM Fig. 4a). This is in line with the predominant CD4+ T cell nature of the immune infiltrate on CT.
Normal T cell proliferation but reduced cytokine production on CT
We next examined whether our CT still permitted functional T cell responses. We could confirm that polyclonal splenocytes recovered from untreated, anti-CD3- or CT-treated animals proliferated readily on restimulation with anti-CD3 or ConA in vitro. Splenocytes from mice treated with CT or anti-CD3 alone displayed some basal proliferation and even had a slightly increased response to ConA stimulation (Fig. 4a). Treatment of islet recipients with anti-CD3 alone increased the production of TNF-α, IL-5, IL-21 and IL-10, but importantly, the upregulation of these cytokines was abrogated by the additional treatment with CsA and TX527 in the CT (Fig. 4b). Moreover, CT reduced IL-2 production, as expected because of inclusion of CsA, and slightly increased IL-17 (Fig. 4b). Overall, we found that in vitro proliferation was not impaired, but cytokine production was skewed in CT-treated islet recipients.
Intact proliferative capacity but reduced cytokine production on CT. (a) On day 21 after transplantation, splenocytes were stimulated in vitro by 1 μg/ml anti-CD3 or 6.25 μg/ml ConA, or remained unstimulated. [3H]Thymidine was added for the last 16 h of the 72 h period. Symbols represent the mean of quadruplicate wells of individual mice, and line and error bars reflect group mean and SEM. (b) On day 21 after transplantation, splenocytes were stimulated in vitro by 6.25 μg/ml ConA for 56 h and cytokines were measured in the supernatant fractions by cytokine bead array. Symbols, lines and error bars reflect individual values, group mean and SEM, respectively. Statistical significance was calculated by Mann–Whitney test; *p < 0.05; **p < 0.01. CTR, control; aCD3, anti-CD3
CT enhances functional Treg numbers
Next, we verified whether CT with anti-CD3, CsA and TX527 affected the abundance of Tregs, as measured by the presence of CD4+FOXP3+ cells. On day 21 after transplantation, we found increased CD4+FOXP3+ T cell frequencies in the blood, spleen and KDLNs on CT, compared with untreated and anti-CD3-treated islet recipients (Fig. 5a–c). Importantly, we detected CD4+FOXP3+ Tregs in the grafted islets of both anti-CD3- and CT-treated mice, as shown by FACS (Fig. 5d) and immunohistochemistry (Fig. 5e).
CT increases FOXP3+CD4+ T cell frequencies. (a–d) On day 21 after transplantation, blood (a), spleen (b), KDLNs (c) and graft (d) were harvested, and the frequency of Tregs was determined as percentage FOXP3+ in the CD4+ fraction using flow cytometry. Symbols, lines and error bars reflect individual values, group mean and SEM, respectively. (e) Detection of FOXP3 (green) and CD4 (blue) in the transplanted islet mass (insulin: red) using immunofluorescence microscopy. (f) CD25-depleted splenocytes (9 × 106) from diabetic NOD mice (white circles; n = 30) were co-transferred with 1 × 106 CD4+CD25+ cells isolated from untreated (white upside-down triangles; n = 5), anti-CD3- (grey squares; n = 5) or CT- (black circles; n = 8) treated transplant-recipient mice (day 21) into 6-week-old NOD Scid mice. Shown is the diabetes incidence (glycaemia >11.1 mmol/l) in recipient mice and the number of mice per group. (g) Dye-labelled CD4+CD25− responder T cells (Tresp) were stimulated with anti-CD3 (1 μg/ml) in the presence of CD4+CD25+ T cells from day-21 CT-treated islet recipients (Treg), APCs and neutralising antibodies (10 μg/ml): anti-CTLA-4 (white triangles); no antibody (black circles); anti-IL-10 (white upside-down triangles). Shown is the percentage of cells that underwent two or more divisions, normalised to Tresp only. (h) Percentage of normoglycaemic mice per group of transplant recipients receiving control treatment (white circles), combination therapy (black triangles) or combination therapy plus anti-CTLA-4 (white triangles) (n = 3). Statistical significance was calculated using the Mann–Whitney non-parametric test (a, d) and Mantel–Cox logrank (c, e): ns, not significant; *p < 0.05; **p < 0.01. CTR, control; aCD3, anti-CD3
We then examined whether the Treg fraction that was increased on CT of islet recipients maintained intact suppressive capacity. In vivo, CD4+CD25+ Tregs isolated from CT-treated islet recipients (CT-Tregs) significantly delayed diabetes in the NOD Scid transfer model (Fig. 5f). CT-Tregs were also more effective than Tregs present in control or anti-CD3-treated islet recipients (Fig. 5f). In vitro, CT-Tregs suppressed the proliferation of CD4+CD25− responder T cells from normoglycaemic NOD mice (Fig. 5g). Moreover, blocking of CTLA-4 and not IL-10 abrogated the in vitro suppressive capacity, indicating that the CT-induced Tregs acted in a CTLA-4-dependent manner, at least in vitro (Fig. 5g). To test whether the CT depended on CTLA-4 to delay diabetes recurrence after syngeneic islet transplantation, we treated islet recipients with blocking antibodies to CTLA-4 (clone UC10-4F10) during CT. This showed that CT immune regulation is at least partly dependent on CTLA-4 (Fig. 5h).
We conclude that combination therapy of syngeneic islet recipients induces functional Tregs, which act in a CTLA-4-dependent manner.
Discussion
We tested a novel combination immunotherapy in severely diabetic NOD mice transplanted with a functional syngeneic islet mass. The importance of choosing this approach over the standard approach with recent-onset or at-onset diabetic NOD mice is the opportunity for a targeted assessment of the effect of immune-directed therapy on the ongoing autoimmune responses independently of a variable presence of residual functional beta cell mass. Indeed, the success of any therapy in diabetic NOD mice is most certainly a function of two variables: (1) dampening of the autoimmune response; (2) sufficient insulin production by the remaining beta cell mass. It is thus conceivable that immunotherapy performs perfectly in terms of dampening or redirecting autoimmune responses, but that this effect is not appreciated because the insufficient beta cell mass does not allow glycaemia normalisation. Introduction of a functional beta cell mass eliminates this confounding variable.
Our CT unites the immunomodulatory actions of anti-CD3 with CsA and a bioactive vitamin D3 analogue (TX527), all used at sub-therapeutic doses to prevent dose-dependent adverse events. Indeed, besides avoiding generalised immune suppression as evidenced by the intact mitogen-driven proliferative capacity of splenocytes from CT-treated mice, our CT did not result in severe hypercalcaemia and bone turnover as evidenced by acceptable serum calcium, osteocalcin and bone calcium concentrations at the end of the follow-up period. Thus, our triple CT produced safe and significantly prolonged grafted islet preservation and, while not a permanent disease remission, extended the diabetes-free period long (up to 40 days) after cessation of the treatment, far better than the diabetes-free period in any of the monotherapy or dual-therapy groups tested (even with higher doses), which already relapsed during the course of treatment.
High-dose anti-CD3 as whole mAb or F(ab')2 fragments can cause an apparent elimination of T cells, because of antigenic modulation of the TCR–CD3 complex [4, 29], physical T cell depletion [3, 4], or altered trafficking [30, 31]. Despite the lower doses of anti-CD3 mAb used in this study (cumulative dose per mouse 12.5 μg whole IgG clone 145-2C11 over 5 days), we also observed a partial decrease in the CD4+ T cell frequency in the spleen and KDLNs of anti-CD3- or CT-treated animals. In line with previous data [4], this phenomenon was transient and normalised by day 21 after transplantation. However, the replenishing of the depleted T cell pool coincides with the destruction of the transplanted islet mass in anti-CD3-treated islet recipients, suggesting that anti-CD3 alone is insufficient to restrain the autoreactivity of the homeostatically expanded T cells, a problematic side effect of immunosuppressive therapies [32, 33]. Our CT on the other hand resembles anti-CD3 monotherapy in its partial and transient T cell depletion, suggesting that additional phenomena must explain the prolonged diabetes-free period.
First, CT limited the infiltration of islets by CD8+ T cells in general and effector/memory CD8+ T cells in particular, which are well established as primary mediators of beta cell destruction in type 1 diabetes [34]. Second, co-administration of TX527 and CsA abrogated the splenocyte-derived production of proinflammatory cytokines, TNF-α, IL-5 and IL-21, induced by anti-CD3 treatment alone. IL-21 has been shown to have a critical role in type 1 diabetes development in NOD mice [26], and neutralisation of IL-21 delayed diabetes recurrence upon syngeneic islet transplantation [35]. IL-10, an immunomodulatory cytokine produced by Tr1 cells [36], was not produced by splenocytes from CT-treated mice, suggesting that IL-10 is not required for the efficacy of the CT. This was further supported by the IL-10-independent suppression by CD4+CD25+ Tregs derived from CT-treated mice. Third, the unique ability of anti-CD3 mAbs to induce long-term disease remission in diabetic mice and individuals has been linked to their capacity to promote induction and/or expansion of Tregs [4, 29, 37, 38]. More specifically, it has been proposed that anti-CD3-mediated T cell depletion preferentially affects the effector T cell compartment, whereas Tregs rather undergo antigenic modulation of the CD3–TCR complex, ultimately giving rise to increased numbers of Tregs [39–42]. Similarly in our study, low-dose anti-CD3 equally depleted CD4+ Tregs and effector T cell subsets early after treatment, but increased Tregs in blood, spleen and KDLNs by day 21 after transplantation. CT further increased the FOXP3+ fraction of CD4+ T cells in the spleen, blood and KDLNs, albeit limited. The CT-induced Tregs were functional because they delayed diabetes in vivo in the NOD Scid transfer model, and suppressed in vitro polyclonal activation in a CTLA-4-dependent manner. Moreover, CT-mediated islet graft preservation depended on CTLA-4, at least in part.
The strength of our CT resides in its potential to target multiple cellular players of the autoimmune response. It is possible that the peripheral increase in Tregs sufficiently interferes with the activation of effector CD8+ T cells in the local lymph nodes and/or their migration to the graft to yield this phenomenon. In addition, the vitamin D3 analogue, TX527, can also modulate APCs [43] and inhibit the release of inflammatory cytokines and chemokines by beta cells under immune attack, thereby limiting the further recruitment of inflammatory immune cells [44]. Unravelling these potential mechanistic pathways is the subject of ongoing and future studies.
We show that CT consisting of sub-therapeutic doses of anti-CD3 mAb, CsA and a non-hypercalcemic vitamin D3 analogue (TX527) restrains autoreactivity in islet-transplanted diabetic NOD mice and prevents recurrent autoimmune responses. Individual agents of the CT clearly cooperate to enhance their individual potency, thereby offering an interesting strategy for circumventing the dose-related side effects of anti-CD3 mAb currently encountered in the treatment of autoimmune diabetes.
Abbreviations
- APC:
-
Antigen-presenting cell
- ConA:
-
Concanavalin A
- CT:
-
Combination therapy
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- CXCR3:
-
CXC chemokine receptor 3
- FOXP3:
-
Forkhead box P3
- H&E:
-
Haematoxylin and eosin
- KDLN:
-
Kidney draining lymph node
- mAB:
-
Monoclonal antibody
- TCR:
-
T cell receptor
- Treg:
-
T regulatory cell
References
Van Belle TL, Coppieters KT, von Herrath MG (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91:79–118
Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464:1293–1300
Chatenoud L, Thervet E, Primo J, Bach JF (1994) Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 91:123–127
Chatenoud L, Primo J, Bach JF (1997) CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158:2947–2954
Herold KC, Hagopian W, Auger JA et al (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698
Herold KC, Gitelman SE, Masharani U et al (2005) A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54:1763–1769
Keymeulen B, Vandemeulebroucke E, Ziegler AG et al (2005) Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608
Keymeulen B, Candon S, Fafi-Kremer S et al (2010) Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood 115:1145–1155
Sherry N, Hagopian W, Ludvigsson J et al (2011) Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 378:487–497
Van Etten E, Decallonne B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C (2003) Analogs of 1alpha,25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem 88:223–226
Penna G, Roncari A, Amuchastegui S et al (2005) Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+FOXP3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 106:3490–3497
Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L (2002) A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374
Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP (2009) Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol 182:6071–6078
van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO (2004) 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun 23:233–239
van Etten E, Dardenne O, Gysemans C, Overbergh L, Mathieu C (2004) 1,25-Dihydroxyvitamin D3 alters the profile of bone marrow-derived dendritic cells of NOD mice. Ann N Y Acad Sci 1037:186–192
Van Belle TL, Gysemans C, Mathieu C (2011) Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab 25:617–632
Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R (1992) 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 41:1491–1495
Mathieu C, Waer M, Casteels K, Laureys J, Bouillon R (1995) Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology 136:866–872
Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R (1994) Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 37:552–558
Gysemans C, van Etten E, Overbergh L et al (2002) Treatment of autoimmune diabetes recurrence in non-obese diabetic mice by mouse interferon-beta in combination with an analogue of 1alpha,25-dihydroxyvitamin-D3. Clin Exp Immunol 128:213–220
Casteels KM, Mathieu C, Waer M et al (1998) Prevention of type I diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology 139:95–102
Casteels K, Waer M, Laureys J et al (1998) Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin D3 analog and cyclosporine. Transplantation 65:1225–1232
Brandt C, Pavlovic V, Radbruch A, Worm M, Baumgrass R (2009) Low-dose cyclosporine A therapy increases the regulatory T cell population in patients with atopic dermatitis. Allergy 64:1588–1596
Zeiser R, Nguyen VH, Beilhack A et al (2006) Inhibition of CD4+CD25+ regulatory T cell function by calcineurin-dependent interleukin-2 production. Blood 108:390–399
Shoda LK, Young DL, Ramanujan S et al (2005) A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 23:115–126
Sutherland APR, van Belle T, Wurster AL et al (2009) Interleukin-21 is required for the development of Type 1 diabetes in NOD mice. Diabetes 58:1144–1155
Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C (2003) The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech 14:33–43
Chatenoud L, Bluestone JA (2007) CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 7:622–632
Mehta DS, Christmas RA, Waldmann H, Rosenzweig M (2010) Partial and transient modulation of the CD3-T cell receptor complex, elicited by low-dose regimens of monoclonal anti-CD3, is sufficient to induce disease remission in non-obese diabetic mice. Immunology 130:103–113
Waldron-Lynch F, Henegariu O, Esplugues E et al (2011) Teplizumab treatment induces migration of human T regulatory lymphocytes to the small intestine in vivo. Diabetes 60:A90
Esplugues E, Huber S, Gagliani N et al (2011) Control of TH17 cells occurs in the small intestine. Nature 475:514–518
Monti P, Scirpoli M, Maffi P et al (2008) Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest 118:1806–1814
Van Belle T, von Herrath M (2008) Immunosuppression in islet transplantation. J Clin Invest 118:1625–1628
Tsai S, Shameli A, Santamaria P (2008) CD8+ T cells in type 1 diabetes. Adv Immunol 100:79–124
McGuire HM, Walters S, Vogelzang A et al (2011) Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60:867–875
Groux H, O'Garra A, Bigler M et al (1997) A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature 389:737–742
Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L (2003) TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9:1202–1208
Kohm AP, Williams JS, Bickford AL et al (2005) Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol 174:4525–4534
Chatenoud L (2010) Immune therapy for type 1 diabetes mellitus: what is unique about anti-CD3 antibodies? Nat Rev Endocrinol 6:149–157
Carpenter PA, Pavlovic S, Tso JY et al (2000) Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J Immunol 165:6205–6213
Wesselborg S, Janssen O, Kabelitz D (1993) Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol 150:4338–4345
Penaranda C, Tang Q, Bluestone JA (2011) Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol 187:2015–2022
van Etten E, Decallonne B, Bouillon R, Mathieu C (2004) NOD bone marrow-derived dendritic cells are modulated by analogs of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 89–90:457–459
Gysemans CA, Cardozo AK, Callewaert H et al (2005) 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 146:1956–1964
Acknowledgements
We thank L. Chatenoud (INSERM, Unité 1013, Université Paris Descartes, Sorbonne Paris Cité, Faculté de Médecine, Paris, France) for kindly providing the anti-CD3 mAb, clone 145-2C11. We are grateful to J. Depovere, M. Gilis, E. Verdrengh and W. Cockx (Clinical Experimental Endocrinology, KU Leuven, Leuven, Belgium) for excellent technical assistance.
Funding
This work was supported by grants from the Fund for Scientific Research Flanders (FWO-Vlaanderen G.0734.10), the University of Leuven (KU Leuven GOA 2009/10), the Belgian Government (Interuniversity Attraction Poles, programme P6/40 of the Belgian Federal Science Policy Office), the European Community's Health Seventh Framework Programme (FP7/2009-2014 under grant agreement 241447 with acronym NAIMIT), and the Juvenile Diabetes Research Foundation (JDRF 17-2011-524). F. Baeke is supported by Interuniversity Attraction Poles Grant P6/40 of the Belgian Federal Science Policy Office. T. Van Belle is the holder of an F+ fellowship from KU Leuven and an EFSD fellowship. T. Takiishi is supported through an SBA fellowship from KU Leuven. C. Mathieu is a clinical researcher and H. Korf a postdoctoral fellow of the FWO-Vlaanderen. C. Gysemans is supported by the European Community's Health Seventh Framework Programme (FP7/2009-2014 under grant agreement 241447 with acronym NAIMIT) and KU Leuven.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
FB, TLVB, LD, HK, TT, JL and CG acquired data. FB, TLVB, CG, and CM interpreted the data. FB, TLVB, HK, LD, JL and TT drafted the article. TLVB, CG and CM revised the manuscript critically for important intellectual content. All authors gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Baeke and T. L. Van Belle contributed equally to this work.
C. Gysemans and C. Mathieu share senior authorship.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM Fig. 1
Increased doses of CsA and/or TX527 prolong graft acceptance. Diabetic NOD mice received 500 syngeneic islets from 14- to 21-day old NOD pups together with indicated treatment regimens. Dashed line, untreated; triangles, 7.5 mg/kg/d CsA (day −1 until day 20); circles, 5 μg/kg/d TX527 (day −1 until day 30); squares, CsA+TX527. Shown is the percentage of normoglycemic mice per group as a measure of graft functionality. (PDF 21 kb)
ESM Fig. 2
CT and anti-CD3 mono-therapy reduces intragraft mRNA amounts of Il2, Ifng, TNFa and Il10. Islet grafts were retrieved 10 or 21 days after transplantation and the amounts of the indicated cytokines were measured by real-time RT-PCR. Displayed is the 2−ΔΔCt ratio of indicated target gene using the average Ct values of Actb (β-actin), Hmbs and Rpl27 for normalization, as group mean ± SEM (n = 3). Statistical significance was calculated by Student t-test, ns: not significant; *P < 0.05; **P < 0.01. (PDF 37 kb)
ESM Fig. 3
Low-dose anti-CD3 mAb treatment increases relative fraction of FOXP3+ cells. NOD mice were treated with anti-CD3 mAb (2.5 μg/d i.v., d0-4) and FOXP3+CD4+ T cells were measured in blood on indicated days. a Percentage of FOXP3+ cells in the viable CD4+ T cell gate. b Percentage of FOXP3+CD4+ T cells in the viable cell gate. Symbols represent individual mice, lines connect group averages. (PDF 35 kb)
ESM Fig. 4
Anti-CD3 alone and CT alter the expression of chemokine expression on T cells. On day 10 (a) or day 21 (b) after transplantation, spleens were harvested and the frequency of CCR5+ (top), CXCR3+ (middle) or CXCR6+ (bottom) cells was determined in the CD4+ (left) and CD8+ (right) fraction by flow cytometry. Bars and cross-bars indicate mean ± SEM of the indicated treatment group. Statistical significance between two groups was calculated using Student t-test (ns: not significant, *P < 0.05, **P < 0.01). (PDF 51 kb)
ESM Table 1
Prolonged graft acceptance upon CT with low dose anti-CD3 mAb, CsA and the vitamin D analog TX527. Data are expressed as mean survival time (MST) ± SEM of functioning grafts. ()*: died with functioning grafts; vs : versus; aCD3 : anti-CD3 mAb. Statistical significance was calculated by log rank test: p < 0.05 was considered significant, ns: not significant. (PDF 88 kb)
ESM Table 2
Transient low-grade hypercalcemia upon CT with low-dose anti-CD3, CsA, and the vitamin D analog TX527. Data are expressed as mean ± SEM. N.D.: no data. Statistical significance was calculated by t-test: p < 0.05 was considered significant, ns: not significant. (PDF 11 kb)
Rights and permissions
About this article
Cite this article
Baeke, F., Van Belle, T.L., Takiishi, T. et al. Low doses of anti-CD3, ciclosporin A and the vitamin D analogue, TX527, synergise to delay recurrence of autoimmune diabetes in an islet-transplanted NOD mouse model of diabetes. Diabetologia 55, 2723–2732 (2012). https://doi.org/10.1007/s00125-012-2630-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2630-1