Abstract
Aims/hypothesis
Although cells expressing insulin are detected early in human fetal development, islets isolated from fetal pancreases show poor insulin secretory responses to glucose, which may be the result of deficient glucose sensing. We have used dual and triple immunolabelling of human fetal and adult pancreas sections to investigate the presence of proteins that participate in glucose sensing in the pancreatic beta cell, namely glucose transporter 1 (GLUT 1, also known as SLC2A1), glucose transporter 2 (GLUT2, also known as SLC2A2), glucokinase (GCK) and inwardly rectifying K+ channel (KIR6.2, also known as KCNJ11) and sulphonylurea receptor 1 (SUR1, also known as ABCC8) subunits of ATP-sensitive K+ channels (\( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels).
Materials and methods
Pancreases obtained with ethical approval from human fetuses from 11 to 36 weeks of gestation, from infants and from adults were formalin-fixed and embedded in paraffin. Sections were labelled with antibodies to proteins of interest. Co-production of antigens was examined by dual and triple immunolabelling.
Results
GLUT2 and \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel labelling was detected in the 11-week pancreas, but largely within the pancreatic epithelium, whereas no labelling for GLUT1 was observed. From 15 weeks, GLUT1, GCK and \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel labelling was detected in an increasing proportion of insulin-positive cells and epithelial labelling with \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel antibodies diminished. GLUT2 was seen in the majority of beta cells only after 7 months of age.
Conclusions/interpretation
The results demonstrate that only a subpopulation of beta cells in the human fetal pancreas produce all key elements of the glucose-sensing apparatus, which may contribute to poor secretory responses in early life.
Similar content being viewed by others
Introduction
During fetal and neonatal life, pancreatic beta cells display relatively poor insulin secretory responses to glucose. Isolated human fetal islet preparations show little glucose-stimulated insulin release prior to 17 weeks of gestation; small monophasic responses are seen between 17 and 20 weeks of gestation, but mature biphasic insulin release is only detectable after birth [1]. These observations suggest that fetal beta cells use different regulatory mechanisms from those of mature islets and that there are progressive changes in the expression or activity of key elements of glucose sensing and/or exocytotic machinery during human fetal pancreas development. The precise nature of these changes is poorly understood.
Stimulus–secretion coupling by glucose requires the generation of a metabolic signal that is dependent on glucose transport into the beta cell by glucose transporters GLUT1 (also known as SLC2A1) and/or GLUT2 (also known as SLC2A2) and phosphorylation of glucose by glucokinase (GCK). mRNAs for GLUT1, GLUT2 and GCK are detected in the human fetal pancreas as early as 13 weeks of gestation [2]. ATP generated from glucose metabolism causes beta cell depolarisation through closure of ATP-sensitive K+ channels (\( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels) comprising two subunits, the inward rectifying K+ channel (KIR6.2, also known as KCNJ11) and the sulfonylurea receptor 1 SUR1 (also known as ABCC8). Active \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels have been reported in the human fetal pancreas, although localisation to beta cells was not formally demonstrated [3]. Beta cell depolarisation leads to an influx of extracellular Ca2+ through voltage-dependent calcium channels, which initiates exocytosis of insulin by the activation of calcium-dependent processes. There is little information on the localisation of these key elements of beta cell glucose sensing in human fetal pancreas development. The aim of this study was to investigate the developmental timing of GLUT1, GLUT2, GCK, SUR1 and KIR6.2 production within beta cells to determine whether beta cell deficiency of one or more of these proteins could contribute to poor glucose-stimulated insulin secretion in the human fetus.
Materials and methods
Human pancreases from fetuses with gestational ages of 11, 12, 14, 15 (two specimens), 16 (two specimens), 18 (three specimens), 19 (two specimens), 20, 21, 23, 24, 33 and 36 weeks, from infants of 3 weeks, 6 months and 7.5 months of age and from one child and nine adults were obtained with maternal consent from pregnancy terminations with ethical approval from the Institute of Child Health (University College London), the UK Medical Research Council Tissue Bank, Hammersmith, King’s College Hospital, and from archival material at the University of Oxford and the Municipal Hospital, Reutlingen, Germany. Pancreases were fixed overnight in 4% formalin, wax embedded and sections were cut onto slides for immunohistochemical localisation of beta cell proteins. Sections were dewaxed and rehydrated, then incubated overnight with 1:100 dilutions of primary antibodies to GLUT1, GLUT2, GCK, KIR6.2 and SUR1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Sections to be labelled with GLUT2 or SUR1 antibodies were treated with trypsin for epitope retrieval prior to incubation. Sections to be labelled with GLUT1 and GCK were heated in 10 mmol/l citrate buffer (pH 6) in a microwave twice for 5 min for epitope retrieval. Antibody labelling was visualised using peroxidase-conjugated streptavidin–biotin complexes with diaminobenzidine substrate (DakoCytomation, Ely, UK) or by immunofluorescence using fluorescein isothiocyanate (FITC)- or Texas Red-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA). Identification of cells on sections was performed by subsequent immunofluorescence labelling with 1:50 dilutions of antibodies to glucagon (GCG) (mouse monoclonal; Sigma, Poole, Dorset, UK), somatostatin (SST) (rabbit polyclonal; DakoCytomation), insulin (mouse monoclonal [Sigma] or guinea pig polyclonal [Dakocytomation]) or cytokeratin-19 (CK19, also known as KRT19) (DakoCytomation). CK19 labelling required heat treatment for epitope retrieval. Control experiments demonstrated that, at the intensity of labelling used in the experiments, diaminobenzidine precipitates did not interfere with immunofluorescence labelling as a result of quenching effects. Images of labelled sections were acquired using a digital camera (Micropublisher 5.0; Qimaging, Burnaby, BC, Canada) attached to a fluorescence microscope (TE1000-U; Nikon Instruments, Kingston-upon-Thames, UK), were overlaid and the proportion of insulin-positive cells expressing the protein of interest was counted with the aid of ImageJ image analysis software (http://rsb.info.nih.gov/ij/). The statistical significance of changes in the proportions of cells labelled with antibodies was tested by one-way analysis of variance.
Results
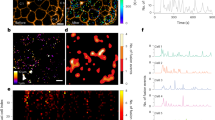
Formalin-fixed paraffin-embedded sections of human fetal pancreas were used to evaluate the production of key proteins involved in the beta cell glucose stimulus-secretion pathway in the human fetal pancreas. The proportions of beta cells labelled for glucose transporters GLUT2 and GLUT1 showed significant increases during pancreatic development (p < 0.001). GLUT2 was detected in the fetal pancreas from 11 weeks but largely within the pancreatic epithelium with very few endocrine cells positive (Fig. 1a), the proportion of beta cells producing the protein remaining low until at least 7 months after birth (Fig. 2). Co-labelling experiments indicated that GLUT2 was present in cells positive for the duct marker CK19, as well as hormone- and CK19-negative cells at the boundary of the epithelium and mesenchyme (Fig. 1b). The majority of beta cells were positive for GLUT2 in the adult (Figs. 1c,d and 2). GLUT2 was also present in small ducts in the adult pancreas (peripheral cell in Fig. 1c). GLUT1 was not detected in the fetal pancreas until 15 weeks of gestation, when the protein was detected in a minority of beta cells. By 16–20 weeks, the majority of beta cells were GLUT1-positive (Figs. 1e,f and 2) and >90% of beta cells were positive for GLUT1 from 20 weeks of gestation to adulthood (Figs. 1g,h and 2). Similarly to GLUT1, GCK was not detected in the human fetal pancreas prior to 15 weeks of gestation, from which time a high proportion of beta cells produced the protein (Figs. 1i–l and 2).
Detection of GLUTs, GCK and \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels in human fetal pancreas development. a–h GLUT2 or GLUT1 in fetal and adult pancreas was detected by immunolabelling and the same sections were co-labelled by immunofluorescence for insulin (INS; red in a, d, f, green in h), CK19 (red, b) or GCG (green, a, d). In a, the inverse image of GLUT2 labelling with diaminobenzidine substrate (brown) was colour-inverted to give a blue colour on a black background and merged with the insulin and GCG labelling of the same section. Cells co-labelled for GLUT2 and CK19 appear orange in b. Bright yellow spots in a and b are autofluorescent structures on the sections. Similar experiments were performed with sections co-labelled for GCK (brown, i, k) with insulin (green, j, l); KIR6.2 (m–o) with insulin (green, m, n, p); KIR6.2 with GCG (green, q, r) and SST (red, q, r); KIR6.2 (s) with CK19 (green, t); and SUR1 (u–w) with GCG (v) or insulin (v, x). Cells co-labelled for \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels and insulin appear pale blue in n and purple in v. The colours of each of the labels are indicated by the colour of the text in the figure. mo, months; p/n, postnatal; w, weeks
Beta cell production of GLUTs, GCK and \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels in human fetal pancreas development. Proportions of insulin-positive cells positive for GLUT2 (black bars), GLUT1 (sloping hatched bars), GCK (horizontally hatched bars), KIR6.2 (vertically hatched bars) and SUR1 (white bars) components of \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels were quantified by analysis of images prepared as in Fig. 1. Results are expressed as means ± SEM of pancreases of 11–15 weeks (n = 5), 16–19 weeks (n = 5) and 20–36 weeks (n = 6) of gestation, infant pancreas (3–7.5 months of age; n = 3) and juvenile and adult pancreases (n = 3). mo, months; w, weeks; y, year
The KIR6.2 subunit of the \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel was detected only in a subpopulation of epithelial cells and not in beta cells prior to 16 weeks of gestation (Fig. 1m). From 16 weeks, KIR6.2 was detected in increasing proportions of insulin-positive fetal pancreatic cells (p < 0.001; Fig. 2), predominantly in those organised as cell clusters (Fig. 1n), and in >80% of beta cells postnatally (Figs. 1o,p and 2). KIR6.2 was rarely detected in GCG- or SST-positive cells in the fetus or adults (Fig. 1q,r). Epithelial localisation in the 12-week fetus was confirmed by co-labelling with CK19 antibody (Fig. 1s,t). A similar pattern of \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel labelling was detected with antibodies to SUR1 (Figs. 1u–x and 2).
Discussion
Previous studies, using molecular and electrophysiological approaches, have established the expression of glucose transporters, GCK and functional \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels in the human fetal pancreas from 13 weeks of gestation [2, 3] and it has been generally assumed, but not formally demonstrated, that these glucose-sensing components are localised to beta cells. The experimental data presented here are consistent with the early developmental appearance of GLUT2 and K+ ATP channels, but suggest that these proteins are predominantly localised to pancreatic epithelium, rather than endocrine cells, prior to 15 weeks of gestation. Two antibodies to different subunits of \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels show identical patterns of labelling, making it unlikely that these observations are the consequence of non-specific recognition by antibody of irrelevant antigens. From 15 to 16 weeks of gestation, increasing proportions of beta cells were labelled with \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel antibodies and a high proportion of beta cells were labelled with GLUT1- and GCK-specific antibodies from this time. Perifusion studies with isolated human fetal islet preparations show sustained insulin secretion in response to glucose from only 17 weeks of gestation [2], an age similar to our detection of the major beta cell glucose sensors. Deficiencies in key glucose-sensing components within beta cells may therefore contribute to poor insulin secretion in early fetal life. Although GLUT2 was detected in epithelial structures from 11 weeks of gestation, the majority of beta cells remained negative for GLUT2 until >7 months after birth, whereas GLUT1 was detected in the majority of beta cells from 16 weeks of gestation. Low levels of beta cell GLUT2 expression were also observed from 12 weeks through to term in a previous study [4]. The techniques used here are not quantitative so do not permit estimation of relative amounts of GLUT1 and GLUT2 in our tissue samples. Nevertheless, the results do suggest that GLUT1 is the major beta cell glucose transporter in fetal and neonatal life and, although we clearly demonstrate that GLUT2 can be detected in the mature pancreas, other studies show that GLUT1 remains the major isoform in the adult pancreas [5, 6].
KIR6.2 and SUR1 labelling was observed in the majority of insulin-positive cells in the adult pancreas, but rarely in GCG- or SST-positive cells. It has been suggested that \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels participate in the regulation of GCG secretion by glucose, since immunohistochemical and electrophysiological studies have detected \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels in rat pancreatic alpha cells [7] and glucose and sulfonylurea effects on GCG secretion are deficient in SUR1 −/− mice [8]. Other studies, in the rat, suggest that only a minority of alpha cells have functional \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels [9]. Hence, there may be heterogeneity, or species-specific differences, in the number of \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channels expressed in alpha cells, and the contribution of these channels to alpha cell glucose sensing in the human pancreas requires further investigation.
The patterns of GLUT2 and GCK expression in the human fetal pancreas are similar to those observed in the rat. GLUT2 mRNA expression was low in islets isolated from fetal or neonatal rats, but increased to adult levels during the suckling period, whereas GLUT2 protein levels remained low until adulthood [10, 11]. GCK mRNA was already at adult levels by late fetal life [10, 11]. In an immunohistochemical study, GLUT2 was restricted to pancreatic epithelium at early stages of rat fetal development and few beta cells were positive for GLUT2 until gestational day 17 [12]. The significance of GLUT2 and \( {\text{K}}^{ + }_{{{\text{ATP}}}} \) channel production by non-endocrine cells in the fetal pancreas is unclear, but it seems unlikely that these have a glucose-sensing role. It is generally accepted that pancreatic beta cells develop from progenitors localised within the fetal pancreatic duct epithelium. Beta cells have low proliferative capacity and the considerable expansion of the endocrine pancreas in late fetal life is likely to be the consequence of neo-islet formation from ductal precursors rather than expansion of pre-existing beta cells [13]. Endocrine differentiation from ductal precursors is associated with expression of transcription factors including forkhead box A2 (FOXA2), hepatocyte nuclear factor 4 (HNF4A), hepatocyte nuclear factor 4 (HNF1A, also known as TCF1), pancreatic and duodenal homeobox 1 (PDX1), paired box gene 4 (PAX4), paired box gene 6 (PAX6), neurogenin 3 (NEUROG3) and neurogenic differentiation 1 (NEUROD1) [14]. FOXA2 and PDX1 are also implicated in regulation of genes involved in stimulus-secretion coupling in mature beta cells, including GLUT2, GCK, KIR6.2 and SUR1 [15]. Expression of elements of beta cell glucose sensing in fetal pancreatic epithelial cells may therefore be a consequence of transcription factor expression during the process of endocrine differentiation and raises the possibility that these proteins may be valuable markers of endocrine precursors in human islet development.
Abbreviations
- CK19:
-
cytokeratin-19
- GCG:
-
glucagon
- GCK:
-
glucokinase
- GLUT1:
-
glucose transporter 1
- GLUT2:
-
glucose transporter 2
- \( K^{ + }_{{ATP}} \) :
-
ATP-sensitive potassium channel
- KIR6.2:
-
inward rectifying K+ channel
- SST:
-
somatostatin
- SUR1:
-
sulfonylurea receptor 1
References
Otonkoski T, Andersson S, Knip M, Simell O (1988) Maturation of insulin response to glucose during human fetal and neonatal development. Studies with perifusion of pancreatic islet-like cell clusters. Diabetes 37:286–291
Mally MI, Otonkoski T, Lopez AD, Hayek A (1994) Developmental gene expression in the human fetal pancreas. Pediatr Res 36:537–544
Weinhaus AJ, Tabiin MT, Poronnik P, Palma CA, Cook DI, Tuch BE (2003) Insulin secretagogues, but not glucose, stimulate an increase in [Ca2+]i in the fetal human and porcine β-cell. J Clin Endocrinol Metab 88:2753–2759
Piper K, Brickwood S, Turnpenny LW et al (2004) Beta cell differentiation during early pancreas development. J Endocrinol 181:11–23
De Vos A, Heimberg H, Quartier E et al (1995) Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96:2489–2495
Ferrer J, Benito C, Gomis R (1995) Pancreatic islet GLUT2 glucose transporter mRNA and protein expression in humans with and without NIDDM. Diabetes 44:1369–1374
Suzuki M, Fujikara K, Kotake K, Inagaki N, Seino S, Takata K (1999) Immunolocalization of sulphonylurea receptor 1 in rat pancreas. Diabetologia 42:1204–1211
Gromada J, Ma X, Hoy M et al (2004) ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse alpha-cells. Diabetes 53(Suppl 3):S181–S189
Quesada I, Nadal A, Soria B (1999) Different effects of tolbutamide and diazoxide in α-, β-, and δ-cells within intact islets of Langerhans. Diabetes 48:2390–2397
Tiedge M, Lenzen S (1993) Differential regulation of glucokinase and GLUT2 glucose transporter gene expression in pancreas and liver of neonatal and 16 day old rats. Biochem Mol Biol Int 29:161–166
Garcia-Flores M, Zueco JA, Arenas J, Blazquez E (2002) Expression of glucose transporter-2, glucokinase and mitochondrial glycerol phosphate dehydrogenase in pancreatic islets during rat ontogenesis. Eur J Biochem 269:119–127
Pang K, Mukonoweshuro C, Wong GG (1994) Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci U S A 91:9559–9563
Bouwens L, Lu WG, De Krijger R (1997) Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 40:398–404
Edlund H (1998) Transcribing pancreas. Diabetes 47:1817–1823
Wang H, Gauthier B, Hagenfeldt-Johansson KA, Iezzi M, Wollheim CB (2002) Foxa2 (HNF3b) controls multiple genes implicated in metabolism-secretion coupling of glucose-induced insulin release. J Biol Chem 277:17564–17570
Acknowledgements
C. C. Richardson was supported by a grant from Diabetes UK.
Duality of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richardson, C.C., Hussain, K., Jones, P.M. et al. Low levels of glucose transporters and \( K^{ + }_{{ATP}} \) channels in human pancreatic beta cells early in development. Diabetologia 50, 1000–1005 (2007). https://doi.org/10.1007/s00125-007-0644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0644-x