Abstract
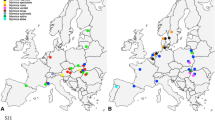
The intensity of interspecific interactions between hosts and symbionts varies among populations of each organism because of differences in the biotic and abiotic environment. We found geographic mosaics in associations between lucanid beetles (Dorcus rectus and Dorcus striatipennis) and symbiotic mites (Haitlingeria sp. and Sandrophela sp., respectively) that were caused by the collapse of host specificity in the northern part of Japan. Haitlingeria sp. was only collected from the surface of the exoskeleton of D. rectus in south and central Japan. Sandrophela sp. showed host specificity in southern to central Japan but was found on both beetle species in areas where Haitlingeria sp. was not found. Because Haitlingeria sp. was able to reproduce on D. rectus collected from Haitlingeria-free regions and no significant differences were observed in average temperature between the host-specific and nonspecific regions bordering on each other, we suggest that the expansion of Haitlingeria sp. in the north has been limited for unknown reasons. When both mites were placed together on D. rectus, only Haitlingeria sp. reproduced, probably because it killed Sandrophela sp., especially juveniles. Thus, we conclude that Sandrophela sp. has expanded its host use to include D. rectus in areas where Haitlingeria sp. is absent. We hypothesise that false host specificity in the canestriniids has been maintained by habitat isolation and/or aggressive behaviour toward competitors. We suggest that host-specific canestriniids provide benefits to hosts that do not develop countermeasures to exclude micro- or macroparasites from their surfaces.


Similar content being viewed by others
References
Augustine L, Muller-Parker G (1998) Selective predation by the mosshead sculpin Clinocottus globiceps on the sea anemone Anthopleura elegantissima and its two algal symbionts. Limnol Oceanogr 43:711–715. doi:10.4319/lo.1998.43.4.0711
Bourke P, Magnan P, Rodriáguez MA (1999) Phenotypic responses of lacustrine brook charr in relation to the intensity of interspecific competition. Evol Ecol 13:19–31. doi:10.1023/A:1006530029418
Brown JM, Wilson DS (1994) Poecilochirus carabi: behavioral and life history adaptations to different hosts and the consequences of geographical shifts in host communities. In: Houck MA (ed) Mites. Chapman and Hall, NY, pp 1–22
Brown SP, Inglis RF, Taddei F (2009) Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evol Appl 2:32–39. doi:10.1111/j.1752-4571,2008.00059.x
De Moraes CM, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570. doi:10.1111/j.0307-6946.2005.00723.x
Dobson AP (1985) The population dynamics of competition between parasites. Parasitology 91:17–347. doi:10.1017/S0031182000057401
Fallon SM, Bermingham E, Ricklefs RE (2005) Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am Nat 165:466–480. doi:10.1086/428430
Fujita H (2010) The lucanid beetles of the world, vol 1. Mushi-Sha, Tokyo
Ganter PF (2006) Yeasts and invertebrate associations. In: Rosa CA, Peter G (eds) Biodiversity and ecophysiology of yeasts. Springer, Verlag, pp 303–370. doi:10.1007/3-540-30985-3_14
Geospatial Information Authority of Japan (1990) The national atlas of Japan. GSI, Tsukuba
Goka K, Kojima H, Okabe K (2004) Biological invasion caused by commercialization of stag beetles in Japan. Glob Environ Res 8:67–74
Hosoya T, Araya K (2005) Phylogeny of Japanese stag beetles (Coleoptera: Lucanidae) inferred from mtrRNA gene sequences with reference to the evolution of sexual dimorphism in mandibles. Zool Sci 22:1305–1318. doi:10.2108/zsj.22.1305
Hosoya T, Honda M, Araya K (2001) Genetic variation of 16S rRNA observed in Ceruchus lignarius and Dorcus rectus rectus (Coleptera: lucanidae). Entomol Sci 4:335–344
Houck MA, OConnor BM (1991) Ecological and evolutionary significance of phoresy in the Astigmata. Ann Rev Entomol 36:611–636. doi:10.1146/annurev.en.36.010191.003143
Hunter PE, Rosario RMT (1988) Associations of Mesostigmata with other arthropods. Ann Rev Entomol 33:393–417. doi:10.1146/annurev.en.33.010188.002141
Japan Meteorological Agency (2012) Climate statistics. http://www.data.jma.go.jp/obd/stats/data/en/index.html. Accessed 11 May 2012
Kanzaki N, Taki H, Masuya H, Okabe K, Tanaka R, Abe F (2011) Diversity of stag beetle-associated nematodes in Japan. Environ Entomol 40:281–288. doi:10.1603/EN10182
Kean RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trend Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Kennedy PG, Peay KG, Bruns TD (2009) Root tip competition among ectomycorrhizal fungi: are priority effects a rule or an exception? Ecology 90:2098–2107. doi:10.1890/08-1291.1
Klimov PB, OConnor BM, Knowles LL (2007) Museum specimens and phylogenies elucidate ecology’s role in coevolutionary associations between mites and their hosts. Evolution 68:1368–1379. doi:10.1111/j.1558-5646.2007.00119.x
Michaels K, Bornemissza G (1999) Effects of clearfell harvesting on lucanid beetles (Coleoptera: Lucanidae) in wet and dry sclerophyll forests in Tasmania. J Insect Conserv 3:85–95. doi:10.1023/A:1009696130694
Moorcroft PR, Pacala SW, Lewis MA (2006) Potential role of natural enemies during tree range expansions following climate change. J Theor Biol 241:601–616. doi:10.1016/j.jtbi.2005.12.019
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878. doi:10.2307/2656184
OConnor BM (1982) Astigmata. In: Parker SP (ed) Synopsis and classification of living organisms, vol 2. McGraw-Hill Book Company, NY, pp 146–169
OConnor BM (2009) Cohort Astigmata. In: Krantz GW, Walter DE (eds) A manual of Acarology. Texas Tech University Press, Lubbock, pp 565–657
Okabe K, Goka K (2008) Potential impacts on Japanese fauna of canestriniid mites (Acari: Astigmata) accidentally introduced with pet lucanid beetles from Southeast Asia. Biodiv Conserv 17:71–81. doi:10.1007/s10531-007-9231-1
Okabe K, Makino S (2008) Parasitic mites as part-time bodyguards of a host wasp. Proc Roy Soc B 275:2293–2297. doi:10.1098/rspb.2008.0586
Pedersen AE, Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trend Ecol Evol 22:133–139. doi:10.1016/j.tree.2006.11.005
Poulin R (2003) The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J Biogeogr 30:1609–1615. doi:10.1111/j.1365- 2699.2005.01288.x
Prenter J, MacNeil C, Dick JTA, Dun AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. doi:10.1016/j.tree.2004.05.002
Schausberger P, Croft BA (2000) Cannibalism and intraguild predation among phytoseiid mites: are aggressiveness and prey preference related to diet specialization? Exp Appl Acarol 24:709–725. doi:10.1023/A:1010747208519
StatSoft Inc (2005) STATISTICA Pro 06J. StatSoft Japan, Tokyo
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–631. doi:10.1038/nature01346
Torchin ME, Byers JE, Huspeni TC (2005) Differential parasitism of native and introduced snails: replacement of a parasite fauna. Biol Invasions 7:885–894. doi:10.1007/s10530-004-2967-6
Walter DE, Lindquist EE, Smith IM, Cook DR, Krantz GW (2009) Order Trombidiformes. In: Krantz GW, Walter DE (eds) A manual of Acarology. Texas Tech University Press, Lubbock, pp 233–420
Wang X-G, Messing RH (2003) Intra- and interspecific competition by Fopius arisanus and Diachasmimorpha tryoni (Hymenoptera: Braconidae), parasitoids of tephritid fruit flies. Biol Control 27:251–259. doi:10.1016/S1049-9644(03)00027-6
Wilkinson HH, Spoerke JM, Parker MA (1996) Divergence in symbiotic compatibility in a legume–bradyrhizobium mutualism. Evolution 50:1470–1477. doi:10.2307/2410884
Woodrings JP (1967) Environmental regulation of andropolymorphism in tyroglyphids (Acari). Proceedings of the 2nd International Congress of Acarology, Budapest, pp 433–440
Yahr R, Vilgalys R, DePriest PT (2006) Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol 171:847–860. doi:10.1111/j.1469-8137.2006.01792.x
Acknowledgments
We thank Goka K, Gotoh T, Itoh K, Inoue T, Iwai N, Kojima H, Kosaka H, Makihara H, Matsumoto K, Mizota K, Nunomura K, Ohsawa M, Okada M, Shimano S, Sugiura S, Suzuki Y, Tanaka E, Tanaka R, Yamaura Y, Yasui Y and Yoshida K for donating the samples. This study was partly supported by a Grant-in-Aid for Scientific Research (B), 2010, #22310145 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Okabe, K., Masuya, H., Kanzaki, N. et al. Regional collapse of symbiotic specificity between lucanid beetles and canestriniid mites. Naturwissenschaften 99, 959–965 (2012). https://doi.org/10.1007/s00114-012-0979-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-0979-0