Abstract
Objective
To assess the effects of propofol treatments at different time points on acute lung injury and on the expression of transforming growth factor (TGF)-β1 and the downstream target of TGF-β1, Smad 2, in the lung tissues in the endotoxic rats.
Methods
Seventy-six Wistar rats were randomly assigned to five groups: control group (saline only), endotoxemic group [lipopolysaccharide (LPS) 8 mg kg−1, i.v.], and three propofol-treated groups. For the propofol-treated groups, propofol (5 mg kg−1, i.v. bolus) was administered either 1 h before LPS, simultaneously with LPS, and 1 h after LPS, and all were followed by infusion of 10 mg kg−1 h−1 of propofol for 5 h after LPS. Lung tissues were sampled to measure myeloperoxidase activity and expression of TGF-β1 and Smad2 and to assess pulmonary microvascular permeability and histopathological changes.
Results
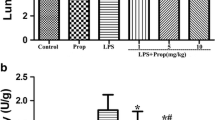
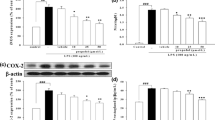
The hemodynamics, arterial blood gases, 5 h survival rate, pulmonary microvascular permeability, and acute lung injury scores were significantly better, and expression of TGF-β1 and Smad2 and myeloperoxidase activity in lung tissues was significantly lower in the pretreatment and simultaneous treatment groups compared to the endotoxemic group. However, there were no significant differences in all observed variables between the endotoxemic and postreatment groups. Except for TGF-β1 expression in lung tissues, the other observed variables were also not significantly different between the pretreatment and simultaneous treatment groups.
Conclusions
In the endotoxic rat model, pretreatment and simultaneous treatment with propofol provided protection against acute lung injury by inhibiting the TGF-β1-Smad2 dependent pathway.





Similar content being viewed by others
References
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–9.
Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–12.
Wesselkamper SC, Case LM, Henning LN, Borchers MT, Tichelaar JW, Mason JM, et al. Gene expression changes during the development of acute lung injury: role of transforming growth factor-β. Am J Respir Crit Care Med. 2005;172:1399–411.
Fahy RJ, Lichtenberger F, McKeegan CB, Nuovo GJ, Marsh CB, Wewers MD. The acute respiratory distress syndrome: a role for transforming growth factor-ß1. Am J Respir Cell Mol Biol. 2003;28:499–503.
Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1ß induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–36.
Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, et al. Involvement of microtubules and Rho pathway in TGF-ß-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–7.
Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rhokinase dependent and independent signals mediate TGF-ß-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol. 2005;288:L294–306.
Shenkar R, Coulson WF, Abraham E. Anti- TGF-β1 monoclonal antibodies prevent lung injury in hemorrhaged mice. Am J Respir Cell Mol Biol. 1994;11:351–7.
Marik PE, Zaloga GP. Therapeutic sedation: has its time come? Crit Care Med. 2002;30:949–52.
Young C, Knudsen N, Andrew H. Sedation in the intensive care unit. Crit Care Med. 2000;28:854–66.
Kwak SH, Choi JI, Park JT. Effects of propofol on endotoxin-induced acute lung injury in rabbit. J Korean Med Sci. 2004;19:55–61.
Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, et al. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann NY Acad Sci. 2005;1042:262–71.
Taniguchi T, Kanakura H, Yamamoto K. Effects of posttreatment with propofol on mortality and cytokine responses to endotoxin-induced shock in rats. Crit Care Med. 2002;30:904–7.
Gao J, Zhao WX, Zhou LJ, Zeng BX, Yao SL, Liu D, et al. Protective effects of propofol on lipopolysaccharide-activated endothelial cell barrier dysfunction. Inflamm Res. 2006;55:385–92.
Takao Y, Mikawa K, Nishina K, Obara H. Attenuation of acute lung injury with propofol in endotoxemia. Anesth Analg. 2005;100:810–6.
Groeneveld ABJ. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vasc Pharm. 2003;39:247–56.
Ware LB, Matthay MB. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49.
Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, et al. TGF-ß is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–44.
Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-ß responses. Cell. 1998;95:737–40.
Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves SMAD2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol. 2006;101:375–84.
Shen X, Li J, Hu PP, Waddell D, Zhang J, Wang XF. The activity of guanine exchange factor NET1 is essential for transforming growth factor-beta-mediated stress fiber formation. J Biol Chem. 2001;276:15362–8.
Green TR, Bennett SR, Nelson VM. Specificity and properties of propofol as an antioxidant free radical scavenger. Toxicol Appl Pharmacol. 1994;129:163–9.
Heller A, Heller S, Blecken S, Urbaschek R, Koch T. Effects of intravenous anesthetics on bacterial elimination in human blood in vitro. Acta Anaesthesiol Scand. 1998;425:518–26.
Taniguchi T, Yamamoto K, Ohmoto N, Ohta K, Kobayashi T. Effects of propofol on hemodynamic and inflammatory responses to endotoxemia in rats. Crit Care Med. 2000;28:1101–6.
Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–7.
Gordon BR, Parker TS, Levine DM, Saal SD, Wang JC, Sloan BJ, et al. Low lipid concentrations in critical illness: implications for preventing and treating endotoxemia. Crit Care Med. 1996;24:584–9.
Hubsch AP, Powell FS, Lerch PG, Doran JE. A reconstituted, apolipoprotein A-I containing lipoprotein reduces tumor necrosis factor release and attenuates shock in endotoxemic rabbits. Circ Shock. 1993;40:14–23.
Baker MT, Naguib M. Propofol: the challenges of formulation. Anesthesiology. 2005;103:860–76.
Acknowledgments
This project was supported in part by a grant from the Natural Science Foundation of Guangdong Province (2005-259). Other funds come from departmental sources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: M. Katori.
J. Gao and F.-S. Xue contributed equally to the writing of the article.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00011-010-0218-0
Rights and permissions
About this article
Cite this article
Gao, J., Zhao, WX., Xue, FS. et al. Early administration of propofol protects against endotoxin-induced acute lung injury in rats by inhibiting the TGF-β1-Smad2 dependent pathway. Inflamm. Res. 59, 491–500 (2010). https://doi.org/10.1007/s00011-009-0110-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0110-y