Abstract
Rapidly growing insight into the molecular biology of colorectal cancer has led to high hopes for the identification of molecular markers to be used in optimized and tailored treatment regimens. However, many of the published data on gene-specific biomarkers are contradictory in their findings, and no tests are currently used in clinical practice, with the exception of microsatellite instability (MSI) and guanylyl cyclase C (GCC) testing in the adjuvant setting, and in Europe KRAS mutation testing is used in the setting of epidermal growth factor receptor (EGFR)-targeted therapy for metastatic disease. There are many reasons for the failure of the initial marker hypothesis-driven approach. Although supported by a good biologic rationale, single markers such as tumor protein p53 (TP53) gene mutations, when applied to a complex tumor type containing many synchronous alterations, do not perform well in predicting outcome. Many markers also suffer from technical shortcomings, resulting from the lack of quantitative techniques to capture the impact of the molecular alteration. The impact of markers obtained from microarray expression profiling needs to be further investigated in studies based on much larger cohorts, and cross-validation studies will be essential.
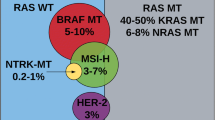
Recently, mutations in the KRAS gene were shown to be strong negative predictors of response to EGFR inhibitors in metastatic disease. It has also been suggested that BRAF gene mutations may be predictive of EGFR inhibitor resistance, and there are some conflicting data regarding the role of the PIK3CA gene. Further studies are needed to help integrate the latest findings into clinically useful tools for personalized medicine.


Similar content being viewed by others
References
Links M, Brown R. Clinical relevance of the molecular mechanisms of resistance to anti-cancer drugs. Expert Rev Mol Med 1999 Oct 25; 1999: 1–21
Schrohl A, Holten-Andersen M, Sweep F, et al. Tumor markers from laboratory to clinical utility. Mol Cell Proteomics 2003; 2: 378–87
McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–4
Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 1996; 88: 1456–66
Kitano H. Cancer as a robust system: implications for anticancer therapy. Nat Rev Cancer 2004; 4(3): 227–35
Allegra CJ, Benedetti JK. Don Quixote and the quest for personalized medicine. J Clin Oncol 2008 Jun 1; 26(16): 2619–20
Loi S, Piccart M, Sotiriou C. The use of gene-expression profiling to better understand the clinical heterogeneity of estrogen receptor positive breast cancers and tamoxifen response. Crit Rev Oncol Hematol 2007; 61(3): 187–94
US FDA Center for Devices and Radiological Health. Draft guidance for industry, clinical laboratories, and FDA staff —in vitro diagnostic multivariate index assays. Document issued 2007 Jul 26 [online]. Available from URL: http://www.fda.gov/cdrh/oivd/guidance/1610.html [Accessed 2008 Dec 18]
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–67
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–52
Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98: 10869–74
Koehler A, Bataille F, Schmid C, et al. Gene expression profiling of colorectal cancer and metastases divides tumours according to their clinicopathological stage. J Pathol 2004; 204: 65–74
D’Arrigo A, Belluco C, Ambrosi A, et al. Metastatic transcriptional pattern revealed by gene expression profiling in primary colorectal carcinoma. Int J Cancer 2005; 115(2): 256–62
Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the WNT pathway. Oncogene 2006; 25: 7531–7
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature 1998; 396: 643–9
WHO. Fact sheet no. 297. Geneva: WHO, 2009 Feb [online]. Available from URL: http://www.who.int/mediacentre/factsheets/fs297/en/print.html [Accessed 2009 Feb 20]
O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004; 96: 1420–5
Ahnen DJ, Feigl P, Quan G, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 1998; 58: 1149–58
Elsaleh H, Powell B, Soontrapornchai P, et al. p53 gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Dukes’ C colon carcinoma. Oncology 2000; 58: 52–9
Westra J, Schaapveld M, Hollema H, et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J Clin Oncol 2005; 23: 5635–43
Roth AD, Tejpar S, Yan P, et al. Correlation of molecular markers in colon cancer with stage-specific prognosis: results of the translational study on the PETACC3-EORTC40993-SAKK60-00 trial [abstract no. 288]. Gastrointestinal Cancers Symposium 2009, American Society of Clinical Oncology; 2009 Jan 15–17; San Francisco (CA)
Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten Ras mutations in patients with colorectal cancer: the multicenter ‘RASCAL’ study. J Natl Cancer Inst 1998; 90: 675–84
Edler D, Glimelius B, Hallstrom M, et al. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol 2002; 20: 1721–8
Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol 1994; 12: 2640–7
Kornmann M, Schwabe W, Sander S, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expression levels: predictors for survival in colorectal cancer patients receiving adjuvant 5-fluorouracil. Clin Cancer Res 2003; 9: 4116–24
Westra JL, Hollema H, Schaapveld M, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase protein expression on survival in adjuvantly treated stage III colon cancer patients. Ann Oncol 2005; 16: 1646–53
Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 1994; 331: 213–21
Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001; 344: 1196–206
Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer 2005; 41: 2060–70
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349: 247–57
Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 2007; 25: 767–72
Alazzouzi H, Alhopuro P, Salovaara R, et al. SMAD4 as a prognostic marker in colorectal cancer. Clin Cancer Res 2005; 11(7): 2606–11
Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 2003; 21: 1174–9
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005; 23(3): 609–18
Birbe R, Palazzo JP, Walters R, et al. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol 2005; 36(2): 170–9
Waldman S, Hyslop T, Schulz S, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA 2009; 301(7): 745–52
Carlson MR. Previstage™ GCC Colorectal Cancer Staging Test: a new molecular test to identify lymph node metastases and provide more accurate information about the stage of patients with colorectal cancer. Mol Diag Ther 2009; 13(1): 11–4
Bertucci F, Salas S, Eysteries S, et al. Gene expression profiling of colon cancer by DNA microarrays and correlation with histoclinical parameters. Oncogene 2004; 23: 1377–91
Wang Y, Jatkoe T, Zhang Y, et al. Gene expression profiles and molecular markers to predict recurrence of Dukes’ B colon cancer. Ann Surg 2004; 22: 1564–71
Barrier A, Boelle P-Y, Roser F, et al. Stage II colon cancer prognosis prediction by tumour gene expression profiling. J Clin Oncol 2006; 24(29): 4685–91
Eschrich S, Yang I, Bloom G, et al. Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol 2005; 23(25): 3526–35
Edler D, Kressner U, Ragnhammar P, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res 2000; 6: 488–92
Santi DV, McHenry CS, Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry 1974; 13: 471–81
Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther 1990; 48: 381–95
Popat S, Chen Z, Zhao D, et al. Prospective, blinded analysis of thymidylate synthase and p53 expression as prognostic markers in the adjuvant treatment of colorectal cancer. Ann Oncol 2006 Dec; 17(12): 1810–7
Marsh S, McKay JA, Cassidy J, et al. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol 2001; 19: 383–6
Allen WL, Johnston PG. Role of genomic markers in colorectal cancer treatment. J Clin Oncol 2005; 23(20): 4545–52
Rodrigues NR, Rowan A, Smith ME, et al. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A 1990; 87: 7555–9
Buglioni S, D’Agnano I, Vasselli S, et al. p53 nuclear accumulation and multiploidy are adverse prognostic factors in surgically resected stage II colorectal cancers independent of fluorouracil-based adjuvant therapy. Am J Clin Pathol 2001; 116: 360–8
Allegra CJ, Paik S, Colangelo LH, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes’ B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 2003; 21: 241–50
Finlay CA, Hinds PW, Tan TH, et al. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol 1988; 8: 531–9
Del Rio M, Molina F, Bascoul-Mollevi C, et al. Gene expression signature in advanced colorectal cancer patients select drugs and response for the use of leucovorin, fluorouracil, and irinotecan. J Clin Oncol 2007; 25(7): 773–80
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987 Jan 9; 235(4785): 177–82
Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 1991 Apr 15; 51(8): 2164–72
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004 May 20; 350(21): 2129–39
Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 2006 Sep 15; 12(18): 5268–72
Amit I, Wides R, Yarden Y. Evolvable signaling networks of receptor tyrosine kinases: relevance of robustness to malignancy and to cancer therapy. Mol Syst Biol 2007; 3: 151
Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005 Mar; 2(3): e73
Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008; 26: 374–9
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–65
Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008; 26(35): 5705–12
Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009 Mar 1; 69(5): 1851–7
Personeni N, Fieuws S, Piessevaux H, et al. Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin Cancer Res 2008 Sep 15; 14(18): 5869–76
Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007 Aug 1; 25(22): 3230–7
Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol 2008 Apr 1; 26(10): 1582–4
Di Fiore F, Van Cutsem E, Laurent-Puig P, et al. Role of KRAS mutations in predicting response and survival in irinotecan-refractory patients treated with cetuximab and irinotecan for metastatic colorectal cancer: analysis of 281 patients with individual data [abstract no. O-016]. Ann Oncol 2008; 19Suppl. 6; vi14
Karapetis C, Khambata-Ford S, Jonker D, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008 Oct 23; 359(17): 1757–65
De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 2008; 19: 508–15
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007; 67: 2643–8
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008 Apr 1; 26(10): 1626–34
Moroni M, Veronese S, Benvenuti S et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 2005; 6: 279–86
Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006; 66: 3992–5
Di Fiore F, Blanchard F, Charbonnier F et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer 2007; 96: 1166–9
Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 2007; 97: 1139–45
Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007; 25: 3230–7
Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009 Apr 2; 360(14): 1408–17
Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009; 27: 663–71
Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009; 360: 563–72
Prenen H, De Schutter J, Jacobs B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res 2009; 15: 3184–8
Acknowledgments
Wendy De Roock is supported by a PhD grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). Sabine Tejpar is Senior Clinical Investigator of the Fund for Scientific Research — Flanders (FWO, Belgium).
The authors have no conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Roock, W., Biesmans, B., De Schutter, J. et al. Clinical Biomarkers in Oncology. Mol Diag Ther 13, 103–114 (2009). https://doi.org/10.1007/BF03256319
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256319