Abstract
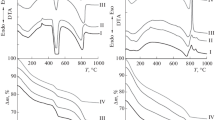
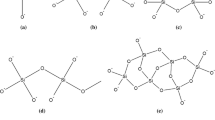
The room-temperature setting process in compacts of various silicate and non-silicate mineral particles bonded with sodium silicate was found to be markedly accelerated by treatment with the acidic gases CO2, S02 and H2S, but was unaffected by neutral or alkaline gases. Strength development increases with gassing time up to a maximum value which depends on the Si to Na ratio of the sodium silicate, the nature of the mineral matter and the gas used. Longer gassing times are needed to achieve ultimate strength with sodium silicates of higher pH and gases of lower solubility in water. The chemical species formed by reaction of CO2, S02 and H2S with sodium silicate were investigated by IR spectroscopy, X-ray diffraction and 29-Si solid state NMR spectroscopy.
Similar content being viewed by others
References
H. J. PEPPLINKHOUSE and W A DAVERN.J. Aust Ceram. Soc. 11 (1975) 42.
M. E TYRRELL. I. L. FELD and J. A BARCLAY.US Bur. Mines Rept. Invest. 7605 (1972)
K. E L. NICHOLS. “The CO2-Silicate Process in Foundnes” (British Cast Iron Research Association, Birmingham. 1972).
K RUSKIN and J. CIHLAR.AFS Int. Cast Met. J 6 (1981) 56.
S. TANIGUCHI, M OHMI, S MATSUBARA and O. HATA,Technol. Rept Osaka Univ. 34 (1984) 1759
K J. D. MACKENZIE, I. W M BROWN and T A. HILL, NZ Patent 212330 (1986).
P. P BERG and N KH IVANOV.Liteinoe Proizvod. (1967) 17.
M. J DAVIES and H. TSUNEI.Kinzoku 42 (1972) 116
M. M FROCHT. “Photoelasticity”, Vol. 2. (Wiley, New York, 1948), p. 121.
M. MÄGI, E LIPPMAA, A SAMOSON, G ENGEL-HARDT and A.-R GRIMMER,J Phys. Chern 88 (1984) 1518.
H. H. W. MOENKE. in “The Infrared Spectra of Minerals”. edited by V. C. Farmer (Mineralogical Society, London. 1974). Ch 16.
R H PIERSON, A. N FLETCHER and E ST CLAIR GANTZ,Anal. Chern. 28 (1956) 1218.
Z NUMAN,Commun. Fac. Sci. Univ. Ankara,A16 (1967) 1
W GIGGENBACH,Inorg. Chem. 10 (1971) 1306.
H H EYSEL, G. WEIGHARDT, H KLEINSCHMAGER and G. WEDDIGEN,Z. Naturforsch. 31B (1976) 415
A. ZIEMANN and W BUES,Z. anorg. allg. Chern.,455 (1979) 69.
“Handbook of Chemistry and Physics”, 62nd Edn (Chemical Rubber Co., Cleveland, Ohio, 1981–2) B73–166.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mackenzie, K.J.D., Brown, I.W.M., Ranchod, P. et al. Silicate bonding of inorganic materials. J Mater Sci 26, 763–768 (1991). https://doi.org/10.1007/BF03163519
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03163519