Abstract
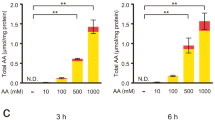
In this study, we evaluate the effects of (−)-epigallocatechin-3-gallate (EGCG) on ultraviolet B (UVB)-irradiated living skin equivalents (LSEs). Histologically, UVB irradiation induced thinning of the LSE epidermis, whereas EGCG treatment led to thickening of the epidermis. Moreover, EGCG treatment protected LSEs against damage and breakdown caused by UVB exposure. Immunohistochemically, UVB-exposed LSEs expressed p53, Fas, and 8-hydroxy-deoxyguanosine (8-OHdG), all of which are associated with apoptosis. However, EGCG treatment reduced the levels of UVB-induced apoptotic markers in the LSEs. In order to determine the signaling pathways induced by UVB, Western blot analysis was performed for both c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), which are associated with UVB-induced oxidative stress. UVB activated JNK in the epidermis and dermis of the LSEs, and EGCG treatment reduced the UVB-induced phosphorylation of JNK. In addition, p38 MAPK was also found to have increased in the UVB-exposed LSEs. Also, EGCG reduced levels of the phosphorylation of UVB-induced p38 MAPK. In conclusion, pretreatment with EGCG protects against UVB irradiationvia the suppression of JNK and p38 MAPK activation. Our results suggest that EGCG may be useful in the prevention of UVB-induced human skin damage, and LSEs may constitute a potential substitute for animal and human studies.
Similar content being viewed by others
References
Ahmed, N. U., Ueda, M., Nikaido, O., Osawa, T., and Ichihashi, M., High levels of 8-hydroxy-2′-deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation.Br. J. Dermatol., 140, 226–231 (1999).
Assefa, Z., Garmyn, M., Bouillon, R., Merlevede, W., Vandenheede, J. R., and Agostinis, P., Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes.J. Invest. Dermatol., 108, 886–891 (1997).
Auger, F. A., Lopez Valle, C. A., Guignard, R., Tremblay, N., Noel, B., Goulet, F., and Germain, L., Skin equivalent produced with human collagen.In Vitro Cell Dev. Biol. Anim. 31, 432–439 (1995).
Bell, E., Ivarsson, B., and Merrill, C., Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potentialin vitro.Proc. Natl. Acad. Sci. U.S.A., 76, 1274–1278 (1979).
Buschmann, T., Potapova, O., Bar-Shira, A., Ivanov, V. N., Fuchs, S. Y., Henderson, S., Fried, V. A., Minamoto, T., Alarcon-Vargas, D., Pincus, M. R., Gaarde, W. A., Holbrook, N. J., Shiloh, Y., and Ronai, Z., Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress.Mol. Cell Biol., 21, 2743–2754 (2001).
Casasco, A., Casasco, M., Zerbinati, N., Icaro Cornaglia, A., and Calligaro, A., Cell proliferation and differentiation in a model of human skin equivalent.Anat. Rec., 264, 261–272 (2001).
Chung, J. H., Han, J. H., Hwang, E. J., Seo, J. Y., Cho, K. H., Kim, K. H., Youn, J. I., and Eun, H. C., Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes.FASEB J., 17, 1913–1915 (2003).
Decraene, D., Agostinis, P., Pupe, A., de Haes, P., and Garmyn, M., Acute response of human skin to solar radiation: regulation and function of the p53 protein.J. Photochem. Photobiol. B., 63, 78–83 (2001).
Decraene, D., Smaers, K., Gan, D., Mammone, T., Matsui, M., Maes, D., Declercq, L., and Garmyn, M., A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytes.J. Invest. Dermatol., 122, 484–491 (2004).
Gensler, H. L., Timmermann, B. N., Valcic, S., Wachter, G. A., Dorr, R., Dvorakova, D., and Alberts, D. S., Prevention of photocarcinogenesis by topical administration of pure epigallocatechin gallate isolated from green tea.Nutr. Cancer, 26, 325–335 (1996).
Hattori, Y., Nishigori, C., Tanaka, T., Uchida, K., Nikaido, O., Osawa, T., Hiai, H., Imamura, S., and Toyokuni, S., 8-Hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure.J. Invest. Dermatol., 107, 733–737 (1996).
Jacobson, M. D., Weil, M., and Raff, M. C., Programmed cell death in animal development.Cell, 88, 347–354 (1997).
Kasai, H., and Nishimura, S., Hydroxylation of deoxy guanosine at the C-8 position by polyphenols and aminophenols in the presence of hydrogen peroxide and ferric ion.Gann, 75, 565–566 (1984).
Katiyar, S. K., Afaq, F., Azizuddin, K., and Mukhtar, H., Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate.Toxicol. Appl. Pharmacol., 176, 110–117 (2001a).
Katiyar, S. K., Afaq, F., Perez, A., and Mukhtar, H., Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress.Carcinogenesis, 22, 287–294 (2001b).
Katiyar, S. K., Ahmad, N., and Mukhtar, H., Green tea and skin.Arch. Dermatol., 136, 989–994 (2000).
Kraemer, K. H., Sunlight and skin cancer: another link revealed.Proc. Natl. Acad. Sci. U. S. A., 94, 11–4 (1997).
Kulms, D., Zeise, E., Poppelmann, B., and Schwarz, T., DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way.Oncogene, 21, 5844–5851 (2002).
Kuo, P. L., and Lin, C. C., Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fasmediated pathways.J. Biomed. Sci., 10, 219–227 (2003).
Lu, Y. P., Lou, Y. R., Xie, J. G., Peng, Q. Y., Liao, J., Yang, C. S., Huang, M. T., and Conney, A. H., Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice.Proc. Natl. Acad. Sci. U. S. A., 99, 12455–12460 (2002).
Mittal, A., Piyathilake, C., Hara, Y., and Katiyar, S. K., Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-3-gallate in hydrophilic cream in SKH-1 hairless mouse model: relationship to inhibition of UVB-induced global DNA hypomethylation.Neoplasia, 5, 555–565 (2003).
Nagata, S., Fas and Fas ligand: a death factor and its receptor.Adv. Immunol., 57, 129–144 (1994).
Nagata, S., and Golstein, P., The Fas death factor.Science, 267, 1449–1456 (1995).
Nanjo, F., Mori, M., Goto, K., and Hara, Y., Radical scavenging activity of tea catechins and their related compounds.Biosci. Biotechnol. Biochem., 63, 1621–1623 (1999).
Naylor, M. F., Erythema, skin cancer risk, and sunscreens.Arch. Dermatol., 133, 373–375 (1997).
Nishigori, C., Hattori, Y., and Toyokuni, S., Role of reactive oxygen species in skin carcinogenesis,Antioxid. Redox. Signal, 6, 561–570 (2004).
Nishigori, C., Wang, S., Miyakoshi, J., Sato, M., Tsukada, T., Yagi, T., Imamura, S., and Takebe, H., Mutations in ras genes in cells cultured from mouse skin tumors induced by ultraviolet irradiation.Proc. Natl. Acad. Sci. U. S. A., 91, 7189–7193 (1994).
Oda, K., Arakawa, H., Tanaka, T, Matsuda, K., Tanikawa, C., Mori, T., Nishimori, H., Tamai, K., Tokino, T., Nakamura, Y., and Taya, Y., p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53,Cell, 102, 849–862 (2000).
Polakowska, R. R., Piacentini, M., Bartlett, R., Goldsmith, L. A., and Haake, A. R., Apoptosis in human skin development: morphogenesis, periderm, and stem cells,Dev. Dyn., 199, 176–188 (1994).
Regnier, M., Asselineau, D., and Lenoir, M. C., Human epidermis reconstructed on dermal substratesin vitro: an alternative to animals in skin pharmacology.Skin Pharmacol., 3, 70–85 (1990).
Rheinwald, J. G., and Green, H., Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells.Cell., 6, 331–343 (1975).
Wang, Z. Y., Agarwal, R., Bickers, D. R., and Mukhtar, H., Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols.Carcinogenesis, 12, 1527–1530 (1991).
Wang, Z. Y., Huang, M. T., Ferraro, T., Wong, C. Q., Lou, Y. R., Reuhl, K., Iatropoulos, M., Yang, C. S., and Conney, A. H., Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice.Cancer Res., 52, 1162–1170 (1992).
Wei, H., Zhang, X., Zhao, J. F., Wang, Z. Y., Bickers, D., and Lebwohl, M., Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas.Free Radic. Biol. Med., 26, 1427–1435 (1999).
Yang, G. Y., Liao, J., Li, C., Chung, J., Yurkow, E. J., Ho, C. T., and Yang, C. S., Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction.Carcinogenesis, 21, 2035–2039 (2000).
Zacchi, V., Soranzo, C., Cortivo, R., Radice, M., Brun, P., and Abatangelo, G.In vitro engineering of human skin-like tissue.J. Biomed. Mater. Res., 40, 187–194 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SY., Kim, DS., Kwon, SB. et al. Protective effects of EGCG on UVB-induced damage in living skin equivalents. Arch Pharm Res 28, 784–790 (2005). https://doi.org/10.1007/BF02977343
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977343