Abstract
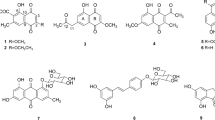
In the course of screening neuraminidase inhibitors from herbal medicines, Reynoutria elliptica exhibited high inhibitory activity. Four active compounds were isolated from the ethyl acetate soluble fraction by consecutive purification using sillica gel, Sephadex LH-20 chromatography, and recrystallization. The chemical structures of these compounds were identified as 1,3,8-trihy-droxy-6-methylanthraquinone (emodin) 1,8-dihydroxy-3-methoxy-6-methylanthraquinone (emodin 3-methyl ether; physcion), 1,3,8-trihydroxy-6-hydoxymethylanthraquinone (ω-hydroxyemodin), and 3,5,4′-trihydroxystilbene (trans-resvertrol) by spectral data including MS,1 H-, and13 C-NMR. The IC50 values of emodin, emodin 3-methyl ether, ω-hydroxyemodin, andtrans-resvertrol were 2.81, 74.07, 10.49, and 8.77 μM, respectively. They did not inhibit other glycosidase such as glucosidase, mannosidase, and galactosidase, indicating that they were relatively specific inhibitors of neuraminidase.
Similar content being viewed by others
References
Burmeister, W. P., Ruigrok, R. W., and Cusack, S., The 2.2 resolution crystal structure of influenza B neuraminidase and its complex with sialic acid.EMBO J., 11, 49–56 (1992).
Chen, L., Han, Y., Yang, F., and Zhang, T., High-speed counter-current chromatography separation and purification resver-trol and piceidPolygonum cuspidatum.J. Chromatogr A., 907, 343–346 (2001).
Colman, P. M., Influenza virus neuraminidase: structure, antibiotics and inhibitors.Protein Sci., 3, 1687–1696 (1994).
Colman, P. M., Design and antiviral properties of influenza virus neuraminidase inhibitors.Pure Appl. Chem., 67, 1683–1688 (1995).
Colman, P. M., A novel approach to antiviral to therapy for influenza.J. Antimicrob. Chemother, 44, 17–22 (1999).
Francis, G. W., Aksnes, D. W., and Holt, Q., Assignment of the1H and13C NMR spectra of anthraquinone glycoside fromRhamnus frangula.Mag. Res. Chem., 36, 769–772 (1998).
Gottschalk, A., The specific enzyme of influenza virus andVibrio cholerae.Biochem. Biophys. Acta., 23, 645–646 (1957).
Klenk, H. O. and Rott, R., The molecular biology of influenza virus pathogenicity.Adv. Virus Res., 34, 247–280 (1988).
Ko, S. K., Whang, W. K., and Kim, I. H., Anthraquinone and stilbene derivatives from the cultivated Korean Rhubarb Rhizomes.Arch. Pharm. Res., 18, 282–288 (1995).
Lin, C., Eichelberger, M. C., Compans, R. W., and Air, G. M., Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly or budding.J. Virol., 69, 1099–1106 (1995).
Likhitwitayawuid, K., Sritularak, B., and De-Eknamkul, W., Tyrosinase inhibitors fromArtocarpus gomezianus.Planta Medica, 66, 275–277 (2000).
Murakami, H., Kobayashi, J., Musuda, T., Morooka, N., and Ueno, Y., ω-Hydroxyemodin, a major hepatic metabolite of emodin in various animals and its mutagenic activity.Mutation Res., 180, 147–153 (1987).
Myers, R. W., Lee, R. T., Lee, Y. C., and Thomas, G. H., The synthesis of 4-methylumberiferyl α-ketoside ofN-acetyl-neuraminic acid and its use in a fluorometric assay for neuraminidase.Anal. Biochem., 101, 166–174 (1980).
Palese, P. and Compans, R. W., Inhibition of influenza virus replication in tissue culture by 2-deoxy-2, 3-dehydro-N-trifluoroacetyl neuraminic acid (FANA): mechanism of action.J. General Virol., 33, 159–163 (1976).
Palese, P., Tabita, U., Ueda, M., and Compans, R. W., Characterization of temperature sensitive influenza virus mutants.Virology, 61, 397–410 (1974).
Varghee, J. N., Mckimm-Breschkin, J. L., Caldwell, J. B., Kortt, A. A., and Colman, P. M., The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor.Protein, 14, 327–332 (1992).
von Itzstein, N., Kok, G. B., and Pegg, M. S., Rational design of potent sialidase-based inhibitors of influenza virus replication.Nature, 363, 418–423 (1993).
Willey, D. C. and Skehel, J. J., The structure and function of the hemagglutinin membrane glycoprotein of influenza virus.Annu. Rev. Biochem., 56, 365–394 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CH., Kim, Sl., Lee, KB. et al. Neuraminidase Inhibitors fromReynoutria elliptica . Arch Pharm Res 26, 367–374 (2003). https://doi.org/10.1007/BF02976693
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02976693