Abstract
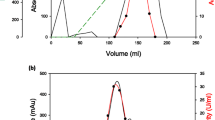
A. niger produced α-glucosidase, α-amylase and two forms of glucoamylase when grown in a liquid medium containing raw tapioca starch as the carbon source. The glucoamylases, which formed the dominant components of amylolytic activity manifested by the organism, were purified to homogeneity by ammonium sulfate precipitation, ion-exchange and two cycles of gel filtration chromatography. The purified enzymes, designated GA1 and GA2, a raw starch digesting glucoamylase, were found to have molar masses of 74 and 96 kDa and isoelectric points of 3.8 and 3.95, respectively. The enzymes were found to have pH optimum of 4.2 and 4.5 for GA1 and GA2, respectively, and were both stable in a pH range of 3.5–9.0. Both enzymes were thermophilic in nature with temperature optimum of 60 and 65°C, respectively, and were stable for 1 h at temperatures of up to 60°C. The kinetic parametersKm andV showed that with both enzymes the branched substrates, starch and amylopectin, were more efficiently hydrolyzed compared to amylose. GA2, the more active of the two glucoamylases produced, was approximately six to thirteen times more active towards raw starches compared to GA1.
Similar content being viewed by others
References
Azizan M.N., Amirul A.-A., Khoo S.L., Najimudin N., Samian R.: Amylolytic activity ofAspergillus nigervan Tieghem: Effect of carbon and nitrogen sources on enzyme production.Biosci. J.4, 1–11 (1993).
Bajpai P., Bajpai P.K.: High temperature alkaline α-amylase fromBacillus licheniformis TCRDC-B13.Biotechnol. Bioeng.33, 72–78 (1989).
Davis B.J.: Disc electrophoresis—II: Method and application to human serum.Ann. N. Y. Acad. Sci.121, 404–427 (1964).
Fairbanks G., Steck T.L., Wallach D.F.H.: Electrophoretic analysis of the major polypeptides of human erythrocyte membrane.J. Biol. Chem.10, 2606–2617 (1971).
Flor P.Q., Hayashida S.: Production and characteristics of raw starch-digesting glucoamylase O from a protease-negative, glycosidase-negativeAspergillus awamori var.kawachi mutant.Appl. Env. Microbiol.45, 905–912 (1983).
Fogarty W.M., Kelly C.T.: Amylases, amyloglucosidases and related glucanases, pp. 115–170 inEconomic Microbiology, Vol. 5. Microbial Enzymes and Bioconversions (A.H. Rose, Ed.). Academic Press, London 1980.
Giulian G.G., Moss R.L., Greaser M.: Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide system.Anal. Biochem.142, 421–436 (1984).
Hayashida S., Kunisaki S., Nakao M., Flor P.Q.: Evidence for raw starch affinity site onAspergillus awamori glucoamylase.Agr. Biol. Chem.46, 83–89 (1982).
Howling D.: Mechanisms of starch enzymolysis.Internat. Biodeterior.25, 15–19 (1989).
Jensen B., Olsen J., Allerman K.: Purification of extracellular enzymes from the thermophylic fungusThermomyces lanuginosus.Can. J. Microbiol.34, 281–223 (1988).
Kanlayakrit W., Ishimatsu K., Nakao M., Hayashida S.: Characteristics of raw-starch-digesting glucoamylase from thermophilicRhizomucor pusillus.J. Ferment. Technol.65, 379–385 (1987).
Khoo S.L., Amirul A.-A., Kamaruzaman M., Nazalan N., Azizan M.N.: Purification and characterization of α-amylase fromAspergillus flavus.Folia Microbiol.39, 392–398 (1994).
Laemmli V.C.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature227, 680–685 (1970).
Lineback D.R., Russell I.J., Rasmussen C.: Two forms of glucoamylase ofAspergillus niger.Arch. Biochem. Biophys.134, 539–553 (1969).
Miah M.N.N., Ueda S.: Multiplicity of glucoamylase ofAspergillus oryzae. Part 2. Enzymatic and physicochemical properties of three forms of glucoamylase.Die Starke29, 235–239 (1977).
Rudick M.J., Elbein A.D.: Glycoprotein enzymes secreted byA. fumigatus: Purification and properties of α-glucosidase.Arch. Biochem. Biophys.161, 281–290 (1974).
Saha B.C., Mitsue T., Ueda S.: Glucoamylase produced by submerged culture ofAspergillus oryzae.Starch/Starke31, 307–314 (1979).
Saha B.C., Ueda S.: Raw starch adsorption, elution and digestion behaviour of glucoamylase ofRhizopus niveus.J. Ferment. Technol.61, 67–72 (1983).
Subrahmanyam A., Mangallan S., Gopalkrishnan K.S.: Amyloglucosidase production byTorula thermophila.Indian J. Exp. Biol.15, 495–496 (1977).
Takahashi T., Tsuchida Y., Irie M.: Purification and some properties of three forms of glucoamylase from aRhizopus species.J. Biochem.84, 1183–1194 (1978).
Takahashi T., Inokuchi N., Irie M.: Purification and characterization of a glucoamylase fromAspergillus saitoi.J. Biochem.89, 125–134 (1981).
Tani Y., Vongsuvankert V., Kumnuanta J.: Raw starch-digestive glucoamylase ofAspergillus sp. N-2 isolated from cassava chips.J. Ferment. Technol.64, 405–410 (1986).
Tosi L.R.O., Terenzi H.F., Jorge J.A.: Purification and characterization of an extracellular glucoamylase from the thermophilic fungusHumicola grisea var.thermoidea.Can. J. Microbiol.39, 846–852 (1993).
Ueda S.: Fungal glucoamylase and raw starch digestion.Trend. Biochem. Sci.6, 89–89 (1981).
Ueda S., Koba Y.: Alcoholic fermentation of raw starch without cooking by using black koji amylase.J. Ferment. Technol.58, 237–242 (1980).
Ueda S., Ohba R., Kano S.: Fractionation of the glucoamylase system from black koji mould and the effects of adding isoamylase and alpha-amylase on amylolysis by the glucoamylase fractions.Die Starke26, 374–378 (1974).
Ueda S., Zenin T., Monteiro D.A., Park Y.K.: Production of ethanol from raw cassava starch by a nonconventional fermentation method.Biotechnol. Bioeng.23, 291–299 (1981).
Yamasaki Y., Suzuki Y.: Purification and properties of α-glucosidase and glycoamylase fromLentinus edodes (Berk.)Sing.Agric. Biol. Chem.42, 971–980 (1977).
Yamasaki Y., Suzuki Y., Ozawa J.: Purification and properties of two forms of glucoamylases fromPenicillium oxalicum.Agric. Biol. Chem.41, 755–762 (1977a).
Yamasaki Y., Suzuki Y., Ozawa J.: Three forms of α-glucosidase and a glucoamylase fromAspergillus awamori.Agric. Biol. Chem.41, 2149–2161 (1977b).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amirul, A.A., Khoo, S.L., Nazalan, M.N. et al. Purification and properties of two forms of glucoamylase fromAspergillus niger . Folia Microbiol 41, 165–174 (1996). https://doi.org/10.1007/BF02814694
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02814694