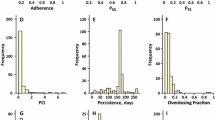
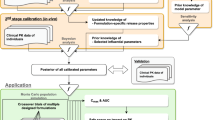
Abstract
For population pharmacokinetic analysis of multiple oral doses one of the key issues is knowing as precisely as possible the dose inputs in order to fit a model to the input-output (dose-concentration) relationship. Recently developed electronic monitoring devices, placed on pill containers, permit precise records to be obtained over months, of the time/date opening of the container. Such records are reported to be the most reliable measurement of drug taking behavior for ambulatory patients. To investigate strategies for using and summarizing this new abundant information, a Markov chain process model was developed, that simulates compliance data from real data from electronically monitored patients, and data simulations and analyses were conducted. Results indicate that traditional population pharmacokinetic analysis methods that ignore actual dosing information tend to estimate biased clearance and volume and markedly overestimate random interindividual variability. The best dosing information summarization strategies consist of initially estimating population pharmacokinetic parameters, using no covariates and only a limited number of dose records, the latter chosen based on an a priori estimate of the half-life of the drug in the compartment of interest; then resummarizing the dose records using either population or individual posterior Bayes parameter estimates from the first population fit; and finally reestimating the population parameters using the newly summarized dose records. Such summarization strategies yield the same parameter estimates as using full dosing information records while reducing by at least 75% the CPU time needed for a population pharmacokinetic analysis.
Similar content being viewed by others
References
M. Davidian and A. R. Gallant. Smooth nonparameteric maximum likehood estimation for population pharmacokinetics, with application to quindine.J. Pharmacokin. Biopharm. 20:529–556 (1992).
L. Aarons. The estimation of population pharmacokinetic parameters using an EM algorithm.Comput. Meth. Prog. Biomed. 41:9–16 (1993).
M. O. Karlsson and L. B. Sheiner. Estimating biovailability when clearnace varies with time.Clin. Pharmacol. Ther. 55:623–637 (1994).
L. P. Balant, M. Rowland, L. Aarons, F. Mentré, P. L. Morselli, J. L. Steimer, and S. Vozeh. New strategies in drug development and clincial evaluation: The population approach.Eur. J. Clin. Pharmacol. 45:93–94 (1993).
New Strategies in Drug Development and Clinical Evaluation The Population Approach, M. Rowland and L. Aarons (eds.). Brussels, Commission of the European Communities, 1992.
L. Aarons. Practical issues in possible implementation of population pharmacokinetics in drug development discussion. In M. Rowland and L. Aarons. (eds.),New Strategies in Drug Development and Clinical Evaluation. The Population Approach, Commission of the European Communities, Brussels, 1992.
M. K. Al-Banna, A. W. Kelman, and B. Whiting. Experimental design and efficient parameter estimation in population pharmacokinetics.J. Pharmacokin. Biopharm. 18:347–360 (1990).
Y. Merlé, F. Mentré, A. Mallet, and A. H. Aurengo. Designing an optimal experiment for bayesian estimation: application to the kinetics of iodine thyroid uptake.Stat. Med. 13:185–196 (1994).
J. Urquhart. Role of patient compliance in clinical pharmacokinetics.Clin. Pharmacokin. 27:202–215 (1994).
Van der Stichele. Measurement of patient compliance and the interpretation of randomized clinical trials.Eur. J. Clin. Pharmacol. 41:27–35 (1991).
D. M. Waterhouse, K. A. Calzone, C. Mele, and D. E. Brenner. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring [see Comments].J. Clin. Oncol. 11:1189–1197 (1993).
S. L. Beal and L. B. Sheiner.NONMEM User's Guide, Part I.NONMEM Project Group (ed.), San Francisco, University of California at San Francisco, 1992.
M. J. Lindstrom and D. M. Bates. Nonlinear mixed effects models for repeated measures data.Biometrics 46:673–687 (1990).
S. L. Beal and L. B. Sheiner.NONMEM User's Guide, Part VII.NONMEM Project Group (ed.), San Francisco, University of California at San Francisco, 1992.
S. M. Ross.Introduction to Probability Models. In Z. W. Birnbaum and C. Lukacs (eds.), Academic Press, New York, 1980.
H. Kastrissios, J. Suarez, B. S. Flowers, and T. F. Blaschke. Could decreased compliance in an AIDS clinical trial affect analysis of outcomes?Clin. Pharmacol. Ther. 57:190 (1995).
Statistical Sciences.S-PLUS Programmer's Manual, Version 3.2, StatSci, a division of MathSoft, Seattle, 1993.
The Coronary Drug Project Research Group. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project.N. Engl. J. Med. 303:1038–1041 (1980).
J. A. Cramer, R. H. Mattson, M. L. Prevey, R. D. Scheyer, and V. L. Quellette. How often is medication taken as prescribed? A novel assessment technique.J. Am. Med. Assoc. 261:3273–3277 (1989).
B. Efron and D. Feldman. Compliance as an explanatory variable in clinical trials.J. Am. Stat. Assoc. 86:9–26 (1991).
D. Gillings. The application of the principle of intention to treat to the analysis of clinical trials.Drug Inform. J. 25:411–424 (1991).
D. B. Rubin. Response to “Compliance as an explanatory variable in clinical trials”.J. Am. Stat. Assoc. 86:36–38 (1991).
G. Levy. A pharmacokinetic perspective on medicament noncompliance.Clin. Pharmacol. Ther. 54:242–243 (1993).
A. Rubio, C. Cox, and M. Weintraub. Prediction of diltiazem plasma concentration curves from limited measurements using compliance data.Clin. Pharmacokin. 22:238–246 (1992).
T. H. Grasela, E. J. Antal, R. J. Townsend, and R. B. Smith. An evaluation of population pharmacokinetics in therapeutic trials. Part I. Comparison of methodologies.Clin. Pharmacol. Ther. 39:605–612 (1986).
E. J. Antal, T. H. Grasela, and R. B. Smith. An evaluation of population pharmacokinetics in therapeutic trials. Part III. Prospective data collection versus retrospective data assembly.Clin. Pharmacol. Ther. 46:552–559 (1989).
Author information
Authors and Affiliations
Additional information
Work supported in part by Cooperative agreements AI 27663 and AI 27666 from the National Institute of Allergy and Infectious Diseases, U.S. Department of Health and Human Services. Dr. Girard's salary supported in part by Grant No. 1 F05 TW05185-01 from the Fogarty International Center, National Institutes of Health, U.S. Department of Health and Human Services.
Rights and permissions
About this article
Cite this article
Girard, P., Sheiner, L.B., Kastrissios, H. et al. Do we need full compliance data for population pharmacokinetic analysis?. Journal of Pharmacokinetics and Biopharmaceutics 24, 265–282 (1996). https://doi.org/10.1007/BF02353671
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02353671