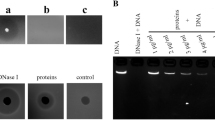
Abstract
Proteolytic activity was found in whole cells and cultural supernatants ofMycoplasma salivarium (PG20 und 8 isolates) andMycoplasma orale 1 (CH19299 and 8 isolates). Additionally, the activity was examined in cell membrane and soluble fractions of PG20 and CH19299, and detected in them. The level of the activity appeared higher inM. salivarium thanM. orale 1. And some differences were found between these mycoplasmas in the affinity for substrates as a result of examination of the activity in cultural supernatants using horse serum, casein and bovine albumin as substrates. That is, PG20 had a higher affinity for horse serum proteins than casein, while CH 19299 the latter than the former, although the lowest affinity for bovine albumin was common to these two strains.
Similar content being viewed by others
References
Aluotto, B. B., Wittler, R. G., Williams, C. O., Faber, J. E.: Standardized bacteriological techniques for the characterization of mycoplasma species. Int. J. Syst. Bact.20, 35–58 (1970)
Czekalowski, W., Hall, D. A., Woolclock, P. R.: Studies of proteolytic activity of mycoplasmas: Gelatinolytic property. J. gen. Microbiol.75, 125–133 (1973)
Freundt, E. A.: The Mycoplasmataceae (The pleuropneumonia group of organisms, morphology, biology and taxonomy). Copenhagen: Munksgaard 1958
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., Randal, R. J.: Protein measurement with the folin phenol reagent. J. biol. Chem.193, 265–273 (1951)
Razin, S., Oliver, Ofra.: Morphogenesis of mycoplasma and bacterial L-form colonies. J. gen. Microbiol.24, 225–237 (1961)
Rodwell, A. W.: Nutrition and metabolism of Mycoplasma mycoides var. mycoides. Ann. N. Y. Acad. Sci.79, 105–116 (1960)
Rodwell, A. W., Abbot, A.: The function of glycerol, choresterol and long-chain fatty acids in the nutrition of Mycoplasma mycoides. J. gen. Microbiol.25, 201–214 (1961)
Smith, P. F., Boughton, J. E.: Role of protein and phospholipid in the growth of pleuropneumonia-like organisms. J. Bact.80, 851–860 (1961)
Watanabe, T., Mishima, K., Horikawa, T.: Proteolytic activity of human mycoplasmas. Jap. J. Microbiol.17, 151–153 (1973)
Watanabe, T.: Proteolytic activity of human oral mycoplasmas. Jap. J. Bact.29, 269 (1974)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watanabe, T. Proteolytic activity ofMycoplasma salivarium andMycoplasma orale 1 . Med Microbiol Immunol 161, 127–132 (1975). https://doi.org/10.1007/BF02121754
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02121754