Abstract
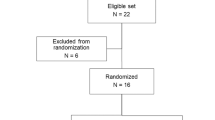
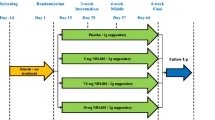
Midodrine is a potent and selective α1-receptor agonist and its potential to increase urethral closure pressure could be useful in the treatment of female stress incontinence. The aim of this randomized double-blind placebo-controlled multicenter study was to evaluate the efficacy and safety of midodrine for the treatment of stress urinary incontinence. The primary criterion of efficacy was the maximum urethral closure pressure at rest. Voiding diaries, symptom and incontinence questionnaires and patient/investigator global assessment were also used to evaluate its efficacy. After 4 weeks of treatment no significant changes in MUCP were found. The global assessment by the patient and investigator did indicate that patients on active treatment had a more positive assessment than the placebo group. In conclusion, midodrine did not cause significant improvements in urodynamic parameters, but there were subjective improvements in some of the patients in the treated groups. Furthermore midodrine was well tolerated.
Similar content being viewed by others
References
Pittner H, Stormann H, Enzenhofer R. Pharmacodynamic actions of midodrine, a new alpha-adrenergic stimulating agent, and its main metabolite ST 1059.Arz Forsch 1976;26:2145–2154
Andersson KE, Ekman G, Henriksson L, Ulmsten U. The effect of midodrine and its active metabolite ST 1059 on the human urethra in vitro and in vivo.Scand J Urol Nephrol 1983;17:261–265
Gnad H, Burmucic R, Petritsch P, Steindorfer P. Conservative treatment of stress incontinence in woman with the α-sympathomimetic midodrine — a double-blind study.Fortschr med 1984;102:578–580
Budde M. Betrachtungen über multiples Testen bei geordneten Alternativen in klinischen Dosisfindungsstudien. In: Bauer P, Hommel G, Sonnemann E, eds. Multiple hypothesis testing. Berlin: Springer, 1988:1–9
Abrams P, Blaivas JG, Stanton SL, Andersen JT. International Continence Society Committee on Standardisation of Terminology. The standardisation of terminology of lower urinary tract function.Scand J Urol Nephrol 1988;(Suppl 114):5–19
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36).Med Care 1992;30:473–483
Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure.Urology 1996;47:67–72
Brooks R. The Euroqol Group. Euroqol, the current state of play.Health Policy 1996;37:53–72
Author information
Authors and Affiliations
Additional information
Editorial Comment: The authors present a controlled trial of midodrine hydrochloride in women with genuine stress incontinence. Although the primary efficacy criterion chosen was maximum urethral pressure at rest, a number of other evaluations were performed, including voiding diaries, subjective symptom questionnaires, and patient/investigator global assessment of therapeutic response. The study finds no change in urethra closure pressure at rest when taking the medication, although both patient and investigator global assessments indicate a significant therapeutic response. The study is ambitious, well accounted for in the report, and the results critically assessed by the authors. Although the results are equivocal as to the efficacy of midodrine hydrochloride in treating stress urinary incontinence, the study clearly presents and discusses the difficulties met in evaluating the pharmacological treatment of incontinence.
Rights and permissions
About this article
Cite this article
Weil, E.H.J., Eerdmans, P.H.A., Dijkman, G.A. et al. Randomized double-blind placebo-controlled multicenter evaluation of efficacy and dose finding of midodrine hydrochloride in women with mild to moderate stress urinary incontinence: a phase II study. Int Urogynecol J 9, 145–150 (1998). https://doi.org/10.1007/BF02001083
Issue Date:
DOI: https://doi.org/10.1007/BF02001083