Abstract
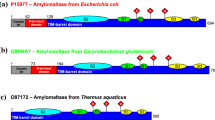
Analysis of the primary structure of mBEII, with those of other branching and amylolytic enzymes as reference, identifies four highly conserved regions which may be involved in substrate binding and in catalysis. When one of the amino acid residues corresponding to the putative catalytic sites of mBEII, i.e., Asp-386, Glu-441, and Asp-509, was replaced, activity disappeared. These putative catalytic residues are located in three different regions (regions 2–4) of the four highly conserved regions (regions 1–4) which exist in the primary structure of most starch hydrolases and related enzymes, including branching enzymes. Region 3, which contains Glu-441 as one of the putative catalytic residues, was located downstream of the carboxyl-terminal position previously reported. The importance of the carboxyl amino acid residues was also demonstrated by chemical modification of the branching enzyme protein using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide.
Similar content being viewed by others
References
Baba, T., Kimura, K., Mizuno, K., Etoh, H., Ishida, Y., Shida, O., and Arai, Y. (1991). Sequence conservation of the catalytic region of amylolytic enzymes in maize branching enzyme-I,Biochem. Biophys. Res. Commun. 181, 87–94.
Bhattacharyya, M., Smith, A. M., Ellis, T. H. N., Hedley, C., and Martin, C. (1990). The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme,Cell 60, 115–122.
Boel, E., Brady, L., Brozozowski, A. M., Derewenda, Z., Dodson, G. G., Jansen, V. J., Petersen, S. B., Swift, H., Thim, L., and Woldike, H. F. (1990). Calcium binding in α-amylases: An X-ray diffraction study at 2.1-Å resolution of two enzymes fromAspergillus, Biochemistry 29, 6244–6249.
Boyer, C. D., and Preiss, J. (1978). Multiple forms of (1,4)-α-D-glucan, (1,4)-α-D-glucan-6-glycosyl transferase from developingZea mays L kernels,Carbohydr. Res. 61, 321–334.
Buisson, G., Duée, E., Haser, R., and Payan, F. (1987). Three dimensional structure or porcine pancreaticα-amylase at 2.9 Å solution. Role of calcium in structure and activity.EMBO J. 6, 3909–3916.
Burnette, W. W. (1981). Western blotting. Electrophoretic transfer of protein from SDS-Polyacrylamide gels to nitrocellulose and radiographic detection with antibody and radiolabeled protein A,Anal. Biochem. 112, 195–203.
Fisher, D. K., Boyer, C. D., and Hannah, L. C. (1993). Starch branching enzyme II from maize endosperm,Plant Physiol. 102, 1045–1046.
Guan, H. P., and Preiss, J. (1993). Differentiation of the properties of the branching isozymes form maize (Zea mays),Plant Physiol. 102, 1269–1273.
Guan, H. P., Baba, T., and Preiss, J. (1994a). Expression of branching enzyme I of maize endosperm inEscherichia coli, Plant Physiol. 104, 1449–1453.
Guan, H. P., Baba, T., and Preiss, J. (1994b). Expression of branching enzyme II of maize endosperm inEscherichia coli, Cell. Mol. Biol. 40, 981–985.
Guan, H. P., Kuriki, T., Sivak, M., and Preiss, J. (1995). Maize branching enzyme catalyzes synthesis of glycogen-like polysaccharide in glgB-deficientEscherichia coli, Proc. Natl. Acad. Sci. USA 92, 964–967.
Hawker, J. S., Ozbun, J. L., Ozaki, H., Greenberg, E., and Preiss, J. (1974). Interaction of spinach leaf adenosine diphosphate glucoseα-1,4 glucanα-4-glucosyl transferase andα-1,4 glucan,α-1,4-glucan-6-glycosyl transferase in synthesis of branchedα-glucan,Arch. Biochem. Biophys. 160, 530–551.
Holm, L., Koivula, A. K., Lehtovaara, P. M., Hemminki, A., and Knowles, J. K. C. (1990). Random mutagenesis used to probe the structure and function ofBacillus stearother-mophilus alpha-amylase,Protein Eng. 3, 181–191.
Holmes, E., Boyer, C., and Preiss, J. (1982). Immunological characterization ofEscherichia coli B glycogen synthase and branching enzyme and comparison with enzymes from other bacteria,J. Bacteriol. 151, 1444–1453.
Jespersen, H. M., MacGregor, E. A., Sierks, M. R., and Svensson, B. (1991). Comparison of the domain-level organization of starch hydrolases and related enzymes,Biochem. J. 280, 51–55.
Jespersen, H. M., MacGregor, E. A., Henrissat, B., Sierks, M., and Svensson, B. (1993). Starch- and glycogen-debranching and branching enzymes: Prediction of structural features of the catalytic (b/a)8-barrel domain and evolutionary relationship to other amylolytic enzymes.J. Protein Chem. 12, 791–805.
Klein, C., and Schulz, G. E. (1991). Structure of cyclodextrin glycosyltransferase refined at 2.0 Å resolution,J. Mol. Biol. 217, 737–750.
Kossmann, J., Visser, R. G. F., Müller-Röber, B. T., Willmitzer, L., and Sonnenwald, U. (1991). Cloning and expression analysis of a potato cDNA that encodes branching enzyme: Evidence for co-expression for starch biosynthetic genes,Mol. Gen. Genet. 203, 237–244.
Kubota, M., Matsuura, Y., Sakai, S., and Katsube, Y. (1991). Molecular structure ofB. stearothermophilus cyclodextrin glucanotransferase and analysis of substrate binding site,Denpun Kagaku 38, 141–146.
Kuriki, T. (1992). Can protein engineering interconvert glucanohydrolases/glucanotransferases, and their specificities?Trends Glycosci. Glycotechonol. 4, 567–572.
Kuriki, T., and Okada, S. (1995). A new concept of the criteria of various amylolytic enzymes and related enzymes; similarities in specificities and structures, inEnzyme Chemistry and Molecular Biology of Amylases and Related Enzymes (lase Research Society of Japan ed.), CRC Press, Boca Raton, Florida, pp. 87–92.
Kuriki, T., Okada, S., and Imanaka, T. (1988). New type of pullulanase fromBacillus stearothermophilus and molecular cloning and expression of the gene inBacillus subtilis, J. Bacteriol. 170, 1554–1559.
Kuriki, T., Takata, H., Okada, S., and Imanaka, T. (1991). Analysis of the active center ofBacillus stearothermophilus neopullulanase,J. Bacteriol. 173, 6147–6152.
Laemmli, U. K. (1970). Cleavage of structure proteins during the assembly of the head bacteriophage T4,Nature 227, 680–685.
Levy, H. M., Leber, P. D., and Ryan, E. M. (1963). Inactivation of myosin by 2,4-dinitrophenol and protection by adenosine triphosphate and other phosphate compounds,J. Biol. Chem. 238, 3654–3659.
Mathupala, S. P., Lowe, S. E., Podkovyrov, S. M., and Zeikus, J. G. (1993). Sequencing of the amylopullulanase (apu) gene ofThermoanaerobactor ethanolicus 39E, and identification of the active site by site-directed mutagenesis,J. Biol. Chem. 268, 16332–16344.
Matsuura, Y., Kusunoki, M., Harada, W., and Kakudo, M. (1984). Structure and possible catalytic residues of Takaamylase A,J. Biochem. (Tokyo)95, 697–702.
Matsuura, Y., Kusunoki, M., and Kakudo, M. (1991). Structure and catalytic implications of Taka-amylase A,Denpun Kagaku 38, 137–139.
Messing, J. (1983). New M13 vectors for cloning,Meth. Enzymol. 101, 20–78.
Mizuno, K., Kawasaki, T., Shimada, H., Satoh, H., Kobayashi, E., Okumura, S., Arai, Y., and Baba, T. (1993). Alteration of the structural properties of starch components by lack of an isoform of starch branching enzyme in rice seeds,J. Biol. Chem. 268, 19084–19091.
Nakamura, A., Haga, K., Ogawa, S., Kuwano, K., Kimura, K. and Yamane, K. (1992). Functional relationship between cyclodextrin glucanotransferase from alkalophilicBacillus andα-amylases. Site-directed mutagenesis of the conserved two Asp and one Glu residues,FEBS Lett. 296, 37–40.
Nakamura, Y., and Yamanouchi, H. (1992). Nucleotide sequence of a cDNA encoding starch-branching enzyme, or Q-enzyme I, from rice endosperm.Plant Physiol. 99, 1265–1266.
Nakamura, Y., Takeichi, T., Kawaguchi, K., and Yamanouchi, H. (1992). Purification of two forms of starch branching enzyme (Q-enzyme) from developing rice endosperm,Physiol. Plant. 84, 329–335.
Plant, A. R., Clemens, R. M., Morgan, H. W., and Daniel, R. M. (1987). Active-site- and substrate-specificity ofThermoanaerobium Tok6-B1 pullulanase,Biochem. J. 246, 537–541.
Podkovyrov, S. M., Burdette, D., and Zeikus, J. G. (1993). Analysis of the catalytic center of cyclomaltodextrinase fromThermoanaerobactor ethanolicus 39E,FEBS Lett. 317, 259–262.
Podkovyrov, S. M., Burdette, D., and Zeikus, J. G. (1993). Analysis of the catalytic center of cyclomaltodextrinase fromThermoanaerobactor ethanolicus 39E,FEBS Lett. 317, 259–262.
Preiss, J. (1991). Biology and molecular biology of starch synthesis and regulation,Oxf. Surv. Plant. Mol. Cell Biol. 7, 59–114.
Romeo, T., Kumar, A., and Preiss, J. (1988). Analysis of theEscherichia coli glycogen gene cluster suggests that catabolic enzymes are encoded among the biosynthetic genes,Gene 70, 363–376.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989).Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
Sanger, F., Nicklen, S., and Coulson. (1977). DNA sequencing with chain-terminating inhibitors,Proc. Natl. Acad. Sci. USA 74, 5463–5467.
Smith, A. M. (1988). Major differences in isoforms of starch-branching enzyme between developing embryos of round and wrinkled-seeded peas (Pisum sativum L.),Planta 175, 270–279.
Smith, P. K., Krohn, R. J., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., and Klenk, D. C. (1985). Measurement of protein using bicinchoninic acid,Anal. Biochem. 150, 76–85.
Svensson, B. (1994). Protein engineering in theα-amylase family: Catalytic mechanism, substrate specificity, and stability.Plant Mol. Biol. 25, 141–157.
Takata, H., Kuriki, T., Okada, S., Takesada, Y., Iizuka, M., Minamiura, N., and Imanaka, T. (1992). Action of neopullulanase. Neopullulanase catalyzes both hydrolysis and transglycosylation atα-(1-4)- andα-(1-6)-glucosidic linkages,J. Biol. Chem. 267, 18447–18452.
Takata, H., Takaha, T., Kuriki, T., Okada, S., Takagi, M., and Imanaka, T. (1994). Properties and active center of thermostable branching enzyme fromBacillus stearothermophilus, Appl. Environ. Microbiol. 60, 3096–3104.
Takeda, Y., Guan, H. P., and Preiss, J. (1993). Branching of amylose by branching isoenzymes of maize endosperm,Carbohydr. Res. 240, 253–263.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuriki, T., Guan, H., Sivak, M. et al. Analysis of the active center of branching enzyme II from maize endosperm. J Protein Chem 15, 305–313 (1996). https://doi.org/10.1007/BF01887119
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01887119