Summary
-
1.
γ-Aminobutyric acid (GABA), a major inhibitory transmitter of the vertebrate retina, is synthesized from glutamate byl-glutamate decarboxylase (GAD) and mediates neuronal inhibition at GABAA receptors. GAD consists of two distinct molecular forms, GAD65 and GAD67, which have similar distribution patterns in the nervous system (Feldblumet al., 1990; Erlander and Tobin, 1991). GABAA receptors are composed of several distinct polypeptide subunits, of which the GABAA α1 variant has a particularly extensive and widespread distribution in the nervous system. The aim of this study was to determine the cellular localization patterns of GAD and GABAA α1 receptor mRNAs to define GABA- and GABAA receptor-synthesizing neurons in the rat retina.
-
2.
GAD and GABAA α1 mRNAs were localized in retinal neurons byin situ hybridization histochemistry with35S-labeled antisense RNA probes complementary to GAD67 and GABAA α1 mRNAs.
-
3.
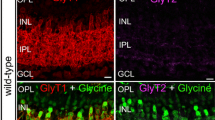
The majority of neurons expressing GAD67 mRNA is located in the proximal inner nuclear layer (INL) and ganglion cell layer (GCL). Occasional GAD67 mRNA-containing neurons are present in the inner plexiform layer. Labeled neurons are not found in the distal INL or in the outer nuclear layer (ONL).
-
4.
GABAA α1 mRNA is expressed by neurons distributed to all regions of the INL. Some discretely labeled cells are present in the GCL. Labeled cells are not observed in the ONL.
-
5.
The distribution of GAD67 mRNA demonstrates that numerous amacrine cells (conventional, interstitial, and displaced) and perhaps interplexiform cells synthesize GABA. These cells are likely to employ GABA as a neurotransmitter.
-
6.
The distribution of GABAA α1 mRNA indicates that bipolar, amacrine, and perhaps ganglion cells express GABAA receptors having anα1 polypeptide subunit, suggesting that GABA acts directly upon these cells.
Similar content being viewed by others
References
Agardh, E., Bruun, A., Ehinger, B., Ekstrom, P., van Veen, T., and Wu, J.-Y. (1987a). Gamma-aminobutyric acid- and glutamic acid decarboxylase-immunoreactive neurons in the retina of different vertebrates.J. Comp. Neurol. 258622–630.
Agardh, E., Ehinger, B., and Wu, J.-Y. (1987b). GABA- and GAD-like immunoreactivity in the primate retina.Histochemistry 86485–490.
Bolz, J., Frumkes, T., Voigt, T., and Wässle, H. (1985). Action and localization ofγ-aminobutyric acid in the cat retina.J. Physiol. (Lond.)362369–393.
Bormann, J. (1988). Electrophysiology of GABAA and GABAB receptor subtypes.Trends Neurosci. 11112–116.
Bowery, N. G., Price, G. W., Hudson, A. L., Hill, D. R., Wilkin, G. P., and Turnbull, M. J. (1984). GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS.Neuropharmacology 23219–231.
Brandon, C. (1985). Retinal GABA neurons: Localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase.Brain Res. 344286–295.
Brandon, C., Lam, D. M. K., and Wu, J.-Y. (1979). Theγ-aminobutyric acid system in rabbit retina: Localization by immunocytochemistry and autoradiography.Proc. Natl. Acad. Sci. USA 763557–3561.
Brecha, N. (1983). Retinal neurotransmitters: Histochemical and biochemical studies. InChemical Neuroanatomy (P. C. Emson, Ed.), Raven Press, New York, pp. 85–129.
Brecha, N., Lai, M., and Sternini, C. (1990). Differential expression of GABAA α1 andα2 receptor mRNAs in the rat retina.Invest. Ophthal. Vis. Sci. Suppl. 31330.
Brecha, N., Johnson, D., Peichl, L., and Wässle, H. (1988). Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase andγ-aminobutyrate immunoreactivity.Proc. Natl. Acad. Sci. USA 856187–6191.
Brecha, N., Sternini, C., Anderson, K., and Krause, J. E. (1989). Expression and cellular localization of substance P/neurokinin A and neurokinin B mRNAs in the rat retina.Vis. Neurosci. 3527–535.
Caruso, D. M., Owczarzak, M. T., Goebel, D. J., Hazlett, J. C., and Pourcho, R. G. (1989). GABA-immunoreactivity in ganglion cells of the rat retina.Brain Res. 476129–134.
Chun, M. H., and Wässle, H. (1989). GABA-like immunoreactivity in the cat retina: Electron microscopy.J. Comp. Neural. 27955–67.
Cox, K. H., DeLeon, D. V., Angerer, L. M., and Angerer, R. C. (1984). Detection of mRNAs in sea urchin embryos byin situ hybridization using asymmetric RNA probes.Dev. Biol. 101485–502.
Erlander, M. G., and Tobin, A. J. (1991). The structural and functional heterogeneity of glutamic acid decarboxylase: A review.Neurochem. Res. 15215–226.
Erlander, M. G., Tillakaratne, N. J. K., Felblum, S., Patel, N., and Tobin, A. J. (1990). Two genes encode distinct glutamate decarboxylases with different responses to pyridoxal phosphate.Soc. Neurosci. Abstr. 16960.
Feldblum, S., Tillakaratne, N. J. K., Erlander, M. G., and Tobin, A. J. (1990). Differential distribution of the mRNAs encoding two forms of glutamic acid decarboxylases in the rat brain: Insights on their functions.Soc. Neurosci. Abstr. 16695.
Grünert, U., and Wässle, H. (1990). GABA-like immunoreactivity in the macaque monkey retina: A light and electron microscopic study.J. Comp. Neurol. 297509–524.
Hendrickson, A., Ryan, M., Noble, B., and Wu, J.-Y. (1985). Colocalization of [3H]muscimol and antisera to GABA and glutamic acid decarboxylase within the same neurons in monkey retina.Brain Res. 348391–396.
Hughes, T. E., Carey, R. G., Vitorica, J., DeBlas, A. L., and Karten, H. J. (1989). Immunohistochemical localization of GABAA receptors in the retina of the new world primateSaimiri sciureus.Vis. Neurosci. 2565–581.
Karschin, A., and Wässle, H. (1990). Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina.J. Neurophysiol. 63860–876.
Khrestchatisky, M., MacLennan, A. J., Chiang, M.-Y., Xu, W., Jackson, M. B., Brecha, N., Sternini, C., Olsen, R. W., and Tobin, A. J. (1989). A novelα-subunit in rat brain GABAA receptors.Neuron 3745–753.
Khrestchatisky, M., MacLennan, A. J., Tillakaratne, N. J. K., Chiang, M.-Y., and Tobin, A. J. (1991). Sequence and regional distribution of the mRNA encoding theα2 polypeptide of rat GABAA receptors.Neurochem. 561717–1722.
Kosaka, T., Tauchi, M., and Dahl, J. L. (1988). Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat.Exp. Brain Res. 70605–617.
Levitan, E. S., Schofield, P. R., Burt, D. R., Rhee, L. M., Wisden, W., Köhler, M., Fujita, N., Rodriguez, H. F., Stephenson, A., Darlison, M. G., Barnard, E. A., and Seeburg, P. H. (1988). Structural and functional basis for GABAA receptor heterogeneity.Nature 33576–79.
Lin, C.-T., Li, H.-Z., and Wu, J.-Y. (1983). Immunocytochemical localization ofl-glutamate decarboxylase, gamma-aminobutyric acid transaminase, cysteine sulfinic acid decarboxylase, aspartate aminotransferase and somatostatin in rat retina.Brain Res. 270273–283.
MacLennan, A. J., Brecha, N., Khrestchatisky, M., Sternini, C., Tillakaratne, N. J. K., Chiang, M.-Y., Anderson, K., Bhakta, K., Lai, M., and Tobin, A. J. (1991). Independent cellular and ontogenetic expression of mRNAs encoding threeα polypeptides of the rat GABAA receptor.J. Neurosci. 43 369–380.
Malherbe, P., Sigel, E., Baur, R., Persohn, E., Richards, J. G., and Möhler, H. (1990). Functional characteristics and sites of gene expression of theα1,β1,γ1-isoforms of the rat GABAA receptor.J. Neurosci. 102330–2337.
Mariani, A. P., and Caserta, M. T. (1986). Electron microscopy of glutamate decarboxylase (GAD) immunoreactivity in the inner plexiform layer of the rhesus monkey retina.J. Neurocytol. 15645–655.
Mariani, A. P., Cosenza-Murphy, D., and Barker, J. L. (1987). GABAergic synapses and benzodiazepine receptors are not identically distributed in the primate retina.Brain Res. 415 153–157.
Möhler, H., Malherbe, P., Draguhn, A., and Richards, J. G. (1990). GABAA-receptors: Structural requirements and sites of gene expression in mammalian brain.Neurochem. Res. 15199–207.
Mosinger, J. L., and Yazulla, S. (1985). Colocalization of GAD-like immunoreactivity and3H-GABA uptake in amacrine cells of rabbit retina.J. Comp. Neurol. 240396–406.
Mosinger, J. L., and Yazulla, S. (1987). Double-label analysis of GAD- and GABA-like immunoreactivity in the rabbit retina.Vision Res. 2723–30.
Mosinger, J. L., Yazulla, S., and Studholme K. (1986). GABA-like immunoreactivity in the vertebrate retina: a species comparison.Exp. Eye Res. 42631–644.
Nishimura, Y., Schwartz, M. L., and Rakic, P. (1985). Localization ofγ-aminobutyric acid and glutamic acid decarboxylase in rhesus monkey retina.Brain Res. 359351–355.
Olsen, R. W., and Venter, J. C. (1986).Benzodiazepine/GABA Receptors and Chloride Channels: Structural and Functional Properties. Receptor Biochemistry and Methodology, Vol. 5, Alan R. Liss, New York.
Olsen, R. W., and Tobin, A. J. (1990). Molecular biology of GABAA receptors.FASEB J. 41469–1480.
Olsen, R. W., McCabe, R. T., and Wamsley, J. K. (1990). GABAA receptor subtypes: Autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system.J. Chem. Neuroanat. 359–76.
Osborne, N. N., Patel, S., Beaton, D. W., and Neuhoff, V. (1986). GABA neurones in retinas of different species and their postnatal developmentin situ and in culture in the rabbit retina.Cell Tissue Res. 243117–123.
Perry, V. H. (1981). Evidence for an amacrine cell system in the ganglion cell layer of the rat retina.Neuroscience 6931–944.
Pourcho, R. G. (1981). Autoradiographic localization of [3H]muscimol in the cat retina.Brain Res. 215187–199.
Pourcho, R. G., and Owczarzak, M. T. (1989). Distribution of GABA immunoreactivity in the cat retina: A light and electron-microscopic study.Vis. Neurosci. 2425–435.
Pritchett, D. B., Sontheimer, H., Shivers, B. D., Ymer, S., Kettenmann, H., Schofield, P. R., and Seeburg, P. H. (1989a). Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology.Nature 338582–585.
Pritchett, D. B., Lüddens, H., and Seeburg, P. H. (1989b). Type I and type II GABAA-benzodiazepine receptors produced in transfected cells.Science 2451389–1392.
Richards, J. G., Schoch, P., Häring, P., Takacs, B., and Möhler, H. (1987). Resolving GABAA/benzodiazepine receptors: Cellular and subcellular localization in the CNS with monoclonal antibodies.J. Neurosci. 71866–1886.
Sarthy, P. V., and Fu, M. (1989a). Localization ofl-glutamic acid decarboxylase mRNA in cat retinal horizontal cells byin situ hybridization.J. Comp. Neurol. 288593–600.
Sarthy, P. V., and Fu, M. (1989b). Localization ofl-glutamic acid decarboxylase mRNA in monkey and human retina byin situ hybridization.J. Comp. Neurol. 288691–697.
Schofield, P. R., Darlison, M. G., Fujita, N., Burt, D. R., Stephenson, F. A., Rodriguez, H., Rhee, L. M., Ramachandran, J., Reale, V., Glencorse, T. A., Seeburg, P. H., and Barnard, E. A. (1987). Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family.Nature 328221–227.
Séquier, J. M., Richards, J. G., Malherbe, P., Price, G. W., Mathews, S., and Möhler, H. (1988). Mapping of brain areas containing RNA homologous to cDNAs encoding theα andβ subunits of the GABAA γ-aminobutyrate receptor.Proc. Natl. Acad. Sci. USA 857815–7819.
Shivers, B. D., Killisch, I., Sprengel, R., Sontheimer, H., Köhler, M., Schofield, P. R., and Seeburg, P. H. (1989). Two novel GABAA receptor subunits exist in distinct neuronal subpopulations.Neuron 3327–337.
Skolnick, P., Paul, S., Zatz, M., and Eskay, R. (1980). “Brain-specific” benzodiazepine receptors are localized in the inner plexiform layer of rat retina.Eur. J. Pharmacol. 66133–136.
Sternini, C., Anderson, K., Frantz, G., Krause, J. E., and Brecha, N. (1989). Expression of substance P/neurokinin A-encoding preprotachykinin messenger ribonucleic acids in the rat enteric nervous system.Gastroenterology 97348–356.
Suzuki, S., Tachibana, M., and Kaneko, A. (1990). Effects of glycine and GABA on isolated bipolar cells of the mouse retina.J. Physiol. (Lond.)421645–662.
Tauck, D. L., Frosch, M. P., and Lipton, S. A. (1988). Characterization of GABA- and glycine-induced currents of solitary rodent retinal ganglion cells in culture.Neuroscience 27193–203.
Vaughn, J. E., Famiglietti, E. V., Jr., Barber, R. P., Saito, K., Roberts, E., and Ribak, C. E. (1981). GABAergic amacrine cells in rat retina: Immunocytochemical identification and synaptic connectivity.J. Comp. Neurol. 197113–127.
Verdoorn, T. A., Draguhn, A., Ymer, S., Seeburg, P. H., and Sakman, B. (1990). Functional properties of recombinant rat GABAA receptors depend upon subunit composition.Neuron 4919–928.
Versaux-Botteri, C., Pochet, R., and Nguyen-Legros, J. (1989). Immunohistochemical localization of GABA-containing neurons during postnatal development of the rat retina.Invest. Ophthal. Vis. Sci. 30652–659.
Wässle, H., and Chun, M. H. (1988). Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina.J. Neurosci. 8 3383–3394.
Wässle, H., and Chun, M. H. (1989). GABA-like immunoreactivity in the cat retina: Light microscopy.J. Comp. Neurol. 27943–54.
Wässle, H., Chun, M. H., and Müller, F. (1987). Amacrine cells in the ganglion cell layer of the cat retina.J. Comp. Neurol. 265391–408.
Wässle, H., Grünert, U., Röhrenbeck, J., and Boycott, B. B. (1989). Cortical magnification factor and the ganglion cell density of the primate retina.Nature 341643–646.
Wisden, W., Morris, B. J., Darlison, M. G., Hunt, S. P., and Barnard, E. A. (1988). Distinct GABAA receptorα subunit mRNAs show differential patterns of expression in bovine brain.Neuron 1937–947.
Wisden, W., Morris, B. J., Darlison, M. G., Hunt, S. P., and Barnard, E. A. (1989). Localization of GABAA receptorα subunit mRNAs in relation to receptor subtypes.Mol. Brain Res. 5305–310.
Yazulla, S. (1986). GABAergic mechanisms in the retina. InProgress in Retinal Research, Vol. 5 (N. N. Osborne and G. J. Chader, Eds.), Pergamon Press, Oxford, pp. 1–52.
Yeh, H. H., Lee, M. B., and Cheun, J. E. (1990). Properties of GABA-activated whole-cell currents in bipolar cells of the rat retina.Vis. Neurosci. 4349–357.
Ymer, S., Schofield, P. R., Draguhn, A., Werner, P., Köhler, M., and Seeburg, P. H. (1989a). GABAA receptorβ subunit heterogeneity: functional expression of cloned cDNAs.EMBO J. 81665–1670.
Ymer, S., Draguhn, A., Köhler, M., Schofield, P. R., and Seeburg, P. H. (1989b). Sequence and expression of a novel GABAA receptorα subunit.FEBS Lett. 258119–122.
Young, W. S., III, and Kuhar, M. J. (1979). Autoradiographic localisation of benzodiapepine receptors in the brains of humans and animals.Nature 280393–395.
Zarbin, M. A., Wamsley, J. K., Palacios, J. M., and Kuhar, M. J. (1986). Autoradiographic localization of high affinity GABA, benzodiazepine, dopaminergic, adrenergic and muscarinic cholinergic receptors in the rat, monkey, and human retina.Brain Res. 37475–92.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brecha, N.C., Sternini, C. & Humphrey, M.F. Cellular distribution ofl-glutamate decarboxylase (GAD) andγ-aminobutyric acidA (GABAA) receptor mRNAs in the retina. Cell Mol Neurobiol 11, 497–509 (1991). https://doi.org/10.1007/BF00734812
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00734812