Abstract
Comparing the properties of ‘young’ and senescent (‘aged’) O+ erythrocytes isolated by applying ultracentrifugation in a self-forming Percoll gradient, we demonstrate that the sialic acids of membrane glycoconjugates control the life span of erythrocytes and that the desialylation of glycans is responsible for the clearance of the aged erythrocytes. This capture is mediated by a β-galactolectin present in the membrane of macrophages. The evidence supporting these conclusions is as follows:
-
(1)
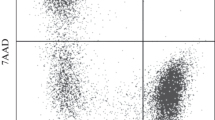
Analysis by flow cytofluorimetry of the binding of fluorescein isothiocyanate labelled lectins specific for sialic acids shows that the aged erythrocytes bind less WGA, LPA, SNA and MAA than young erythrocytes. The binding of DSA and LCA is not modified. On the contrary, the number of binding sites of UEA-I specific for O antigen and of AAA decreases significantly. PNA and GNA do not bind to erythrocytes.
-
(2)
RCA120 as well asErythrina cristagalli andErythrina corallodendron lectins specific for terminal β-galactose residues lead to unexpected and unexplained results with a decrease in the number of lectin binding sites associated with increasing desialylation.
-
(3)
The glycoconjugates from the old erythrocytes incorporate more sialic acid than the young cells. This observation results from the determination of the rate of transfer by α-2,6-sialyltransferase of fluorescent or radioactiveN-acetylneuraminic acid, using as donors CMP-9-fluoresceinyl-NeuAc and CMP-[14C]-NeuAc, respectively.
-
(4)
Microscopy shows that the old erythrocytes are captured preferentially by the macrophages relative to the young ones. Fixation of erythrocytes by the macrophage membrane is inhibited by lactose, thus demonstrating the involvement of a terminal β-galactose specific macrophage lectin.
-
(5)
Comparative study of the binding of WGA, LPA, SNA and MAA to the aged erythrocytes and to thein vitro enzymatically desialylated erythrocytes shows that the desialylation rate of aged cells is low but sufficient to lead to their capture by the macrophages
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- CMP-NeuAc:
-
cytidine monophosphateN-acetylneuraminate
- CSB:
-
cell sialylation buffer
- EDTA:
-
ethylene diamine tetraacetic acid
- FITC:
-
fluoresceinyl isothiocyanate
- 9-FITC-NeuAc:
-
9-fluoresceinyl-N-acetylneuraminate
- NeuAc:
-
N-acetylneuraminic acid
- PAGE:
-
polyacrylamide gel electrophoresis
- PBS:
-
Dulbecco's phosphate buffer saline solution
- PMSF:
-
phenylmethyl-sulfonyl fluoride
- RBC:
-
red blood cells
- SCA:
-
Senescent Cell Antigen
- SDS:
-
sodium dodecyl sulfate
- SFG:
-
senescent factor glycopeptides
- AAA:
-
Aleuria aurantia agglutinin
- DSA:
-
Datura stramonium agglutinin
- ECA:
-
Erythrina cristagalli agglutinin
- GNA:
-
Galanthus nivalis agglutinin
- LCA:
-
Lens culinaris agglutinin
- LFA:
-
Limax flavus agglutinin
- LPA:
-
Limulus polyphemus agglutinin
- MAA:
-
Maackia amurensis agglutinin
- PNA:
-
peanut agglutinin
- RCA:
-
Ricinus communis agglutinin
- SNA:
-
Sambucus nigra agglutinin
- UEA-I:
-
Ulex europeus agglutinin-I
- WGA:
-
Wheat germ agglutinin
References
Schauer R (1982)Adv Carbohydr Chem Biochem 40:131–234.
Aminoff D (1985) InCellular and Molecular Approach of Aging, the Red Cell as a Model, (Eaton JW, Konzen DK, White JG eds), pp. 279–300. New York: Alan R Liss.
Danon D, Marikovsky Y (1988)Blood Cells 14:7–15.
Galili U (1988)Blood Cells 14:205–20.
Aminoff D (1988)Blood Cells 14:229–47.
Lutz HU (1990) InBlood Cell Biochemistry (Harris JR eds), pp. 81–120. New-York: Plenum Press.
Bartosz G (1991)Gerontology 37:33–67.
Garratty G (1991)Gerontology 37:68–94.
Aminoff D, Rolfes-Curl A, Supina E (1992)Arch Gerontol Geriatr, Suppl 3:7–16.
Piomelli S, Seaman C (1993)Am J Hematol 42:46–52.
Kay MMB (1994) InImmunobiology of Transfusion Medicine (Garratty G ed), pp. 173–98, New York: Marcel Dekker.
Kay MMB (1975)Proc Natl Acad Sci USA 72:3521–25.
Lutz HU, Kay MMB (1981)Mech Ageing Develop 15:65–75.
Lutz HU, Flepp R, Stringaro-Wipf G (1984)J Immunol 133:2610–18.
Kay MMB (1984)Proc Natl Acad Sci USA 81:5753–57.
Lutz HU, Bussolino F, Flepp R, Fasler S, Stammler P, Kazatchkine D, Arese P (1987)Proc Natl Acad Sci USA 84:7368–72.
Vaysse J, Gattegno L, Pilardeau P (1992)Eur J Haematol 48:83–86.
Bocci V (1976)Experientia 32:135–40.
Brovelli A, Pallavicini G, Sinigaglia F, Balduini CL, Balduini C (1976)Biochem J 158:497–500.
Khan MT, Wang KW, Villalobo A, Roufagalis BD (1994)J Biol Chem 269:10016–21.
Weed RI, Reed CF (1966)Am J Med 41:6881–98.
Lutz HU (1978)J Supromolec Struct 8:375–89.
Lutz HU (1979)J Biol Chem 25:11177–88.
Schlepper-Schäfer J, Kolb-Bachhofen V (1988)Blood Cells 14:259–69.
Pessina CP, Skiftas S (1983)Int J Biochem 15:277–79.
Stewart WB, Petenyl CW, Rose HM (1955)Blood 10:228–34.
Halbhuber KJ, Helmke U, Geyer G (1972)Folia Haematol 97:196–203.
Jancik J, Schauer R (1974)Hoppe Seyler's Z Physiol Chem 355:395–400.
Jancik J., Andres KH, von Düring M, Schauer R (1978)Cell Tiss Res 186:209–26.
Müller E, Franco MW, Schauer R (1981)Hoppe Seyler's Z Physiol Chem 362:1615–20.
Schlepper-Schäfer J, Kolb-Bachofen V, Kolb H (1983)Biochem Biophys Res Commun 115:551–59.
Aminoff D, Bell WC, Fulton I, Ingebrightsen I (1976)Am J Haematol 1:419–32.
Aminoff D, Vorder-Bruegge WF, Bell WC, Sarpolis K (1977)Proc Natl Acad Sci USA 74:1521–24.
Bell WC, Levy GN, Williams R, Aminoff D (1977)Proc Natl Acad Sci USA 74:4205–9.
Smedsrod B, Aminoff D (1983)Am J Haematol 15:123–33.
Smedsrod B, Aminoff D (1985)Am J Haematol 18:31–40.
Aminoff D, Golstein IJ, Supina E (1991)Glycoconjug J 8:175–76.
Kelm S, Schauer R (1988)Hoppe Seyler's Z Physiol Chem 369:693–704.
Kolb H, Friedrich E, Süss R (1981)Hoppe Seyler's Z Physiol Chem 362:1609–14.
Lutz HU, Stammler P, Fasler S, Ingold M, Fehr J (1992)Biochim Biophys Acta 1116:1–10.
Scatchard G (1949)Ann NY Acad Sci 51:660–72.
Kosa RE, Brossmer R, Gross HJ (1993)Biochem Biophys Res Commun 190:914–20.
Gross HJ, Sticher U, Brossmer R (1990)Anal Biochem 186:127–34.
Linderkamp O, Meiselman HJ (1982)Blood 59:1121–27.
Sorette MP, Galili U, Clark MR (1991)Blood 77:628–36.
Aminoff D, Ghalambor MA, Heinrich CJ (1981) InErythrocyte Membrane (Kruckenberg WC, Eaton JW, Brewer GJ eds) 2. Recent Clinical and Experimental Advances, pp. 269–278, New York: Alan R Liss.
Green GA, Rehn MM, Kabea VK (1985)Blood 65: 1127–33.
Gutowski KA, Hudson JL, Aminoff D (1991)Exp Gerontol 26:315–26.
Rolfes-Curl A, Ogden LL, Omann O, Aminoff D (1991)Exp Gerontol 26:327–45.
Sharon R, Fibach E (1991)Cytometry 12:545–49.
Fibach E, Sharon R (1994)Transfusion 34:328–32.
Cohen NS, Ekholm JE, Luthra MG, Hanahan DJ (1976)Biochim Biophys Acta 419:229–42.
Luner SJ, Szklarek D, Knox RJ, Seaman GVF, Josefowicz JY, Ware BR (1977)Nature 269:719–21.
Gattegno L, Perret G, Fabia F, Cornillot P (1981)Mech Ageing Develop 16:205–19.
Danon D, Marikovsky Y, Skutelsky E (1971) InRed Cell Structure and Metabolism (Ramot B ed) pp. 23–38, New York: Academic Press.
Choy YM, Wong SL, Lee CY (1979)Biochem Biophys Res Commun 91:410–15.
Gattegno L, Bladier D, Garnier M, Cornillot P (1976)Carbohydr Res 52:197–208.
Gattegno L, Fabia F, Bladier D, Cornillot P (1979)Biomedicine 30:194–99.
Bladier D, Gattegno L, Fabia F, Perret G, Cornillot P (1980)Carbohydr Res 83:371–76.
Shinozuka T, Takei S, Yanagida JI, Watanabe H, Ohkuma S (1988)Life Sci 43:683–89.
Gutovski KA, Linseman DA, Aminoff D (1988)Carbohydr Res 178:307–13.
Prokop O, Uhlenbruck G (1969) InHuman Blood and Serum Groups (Prokop O, Uhlenbruck G eds) pp. 103–10.
Tannert C, Schmidt G, Klatt D (1979)Acta Biol Med Germ 38:663–67.
Henrich CJ, Aminoff D (1983)Carbohydr Res 120:55–56.
Vaysse J, Gattegno L, Bladier D, Aminoff D (1986)Proc Natl Acad Sci USA 83:1339–48.
Aminoff D (1988)Glycoconjugate J 5:356.
Aminoff D, Goldstein I J, Supina E (1991)Glycoconjugate J 8:175–76.
Shinozuka T, Takei S, Yanagida J, Watanake H, Ohkuma S (1988)Blut 57:117–23.
Gattegno L, Perret G, Fabia F, Bladier D, Cornillot P (1981)Carbohydr Res 95:283–90.
Gattegno L, Perret G, Felon M, Cornillot P (1982)Comp Biochem Physiol 73B:725–28.
Ghalambor MA, Aminoff D (1979)Fed Proc 38:2235.
Galili U, Korash A, Kahane I, Rachmilewitz EA (1983)Blood 61:1258–64.
Kolb H, Schudt C, Kolb-Bachofen V, Kolb HA (1978)Exp Cell Res 113:319–25.
Kolb H, Kolb-Bachofen V, Schlepper-Schäfer J (1979)Biol. Cell 36:301–8.
Kolb H, Vogt D, Herbertz L, Corfield AP, Schauer R, Schlepper-Schäfer J (1980)Hoppe Seyler's Z Physiol Chem 361:1747–50.
Schlepper-Schäfer J, Kolb-Bachofen V, Kolb H (1980)Biochem J 186:827–31.
Küster JM, Schauer R (1981)Hoppe Seyler's Z Physiol Chem 362:1507–14.
Gattegno L, Saffar L, Vaysse J (1988)Med Sci Res 16:1081–82.
Bennett GD, Kay MMB (1981)Exp Hematol 9:297–307.
Galili U, Korkesh A, Kahane I, Rachmilewitz EA (1983)Blood 61:1258–64.
Lutz H, Stringaro-Wipf G (1984)Biomed Biochim Acta 425:117–21.
Galili U, Flechner E, Knyszynki A, Danon D, Rachmilewitz EA (1986)Br J Haematol 32:317–24.
Gattegno L, Prigent MJ, Saffar L, Bladier D, Vaysse J, Lefloch A (1986)Glycoconjugate J 3:379–89.
Gattegno L, Saffar L, Vaysse L (1989)J Leukoc Biol 45:422–28.
Sheiban E, Gershon H (1993)J Lab Clin Med 121:493–501.
Gattegno L, Bladier D, Vaysse J, Saffar L (1991) InRed Blood Cell Aging (Magnani M, De Flora A, eds) pp. 329–337. New York: Plenum Press.
Bocci V, Pessina GP, Paulesu L (1981)Int J Biochem 13:1257–60.
Bosmann H D (1974)Vox Sang 26:497–512.
Chiarini A, Fiorilli A, Di Francesco L, Venerando B, Tettamanti G (1993)Glycoconjugate J 10:64–71.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bratosin, D., Mazurier, J., Debray, H. et al. Flow cytofluorimetric analysis of young and senescent human erythrocytes probed with lectins. Evidence that sialic acids control their life span. Glycoconjugate J 12, 258–267 (1995). https://doi.org/10.1007/BF00731328
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00731328