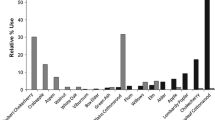
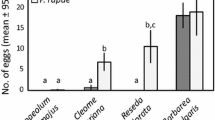
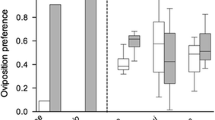
Summary
Early models of hostplant exploitation by phytophagous insects suffer from unwarranted assumptions and may not be generally applicable. Wordmodels of the co-evolutionary approach may assume unwarranted evolutionary stability in ‘strategic’ explanations, whilst mathematical models derived from earlier optimal-diet studies are unrealistic and unwieldy. A simple arithmetic model synthesises these two approaches, using the two parameters of foodplant suitability and availability. Hostplant use by the butterfly Anthocharis cardamines, previously thought to be maladaptively polyphagous, is shown to be optimal under prevailing conditions of short search time. The predictions of the model for hostplant use and community structure of butterflies and other phytophagous insects are tested and, in large part, corroborated. Monophagy and monophagic forms of oligophagy are shown to be favoured by: long adult lifespan; low search costs to females; search images (Whether visual or olfactory); batch-laying of eggs; high differential in foodplant suitability.
Similar content being viewed by others
References
Benson WW (1978) Resource partitioning in Passion Vine butterflies. Evolution 32:493–518
Bowden SR (1970) American white butterflies (Pieridae) and English foodplants. Journal of the Lepidopterists' Society 25:6–12
Chew FS (1975) Coevolution of Pierid butterflies and their cruciferous foodplants. I. The relative quality of available resources. Oecologia 20:117–127
Chew FS (1977a) II. The distribution of eggs on potential foodplants. Evolution 31:568–579
Chew FS (1977b) The effects of introduced mustards (Cruciferae) on some native North American cabbage butterflies (Lep. Pier.) Athala 5:13–19
Courtney SP (1980) Studies on the biology of the butterflies Anthocharis cardamines (L.) and Pieris napi (L.), in relation to speciation in Pierinae. Ph.D. Thessis. University of Durham, England
Courtney SP (1981) Coevolution of Pierid butterflies and their cruciferous foodplants. III. Anthocharis cardamines (L.) survival, development and oviposition on different hostplants. Oecologia 51:91–96
Courtney SP (1982) IV. Hostplant apparency and Anthocharis cardamines oviposition. Oecologia 52:258–265
Ehrlich PR, Raven H (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Emlen JM (1966) The role of time and energy in food preference. Am Nat 100:611–617
Gilbert LE (1975) Ecological consequences of a co-evolved mutualism between butterflies and plants. In: Gilbert and Raven (eds) Coevolution of Animals and Plants. Texas, University Press
Gilbert LE, Singer MC (1975) Butterfly ecology. Annu Rev Ecol System 6:365–397
Hicks KL (1974) Mustard oil glucosides: feeding stimulants for adult Cabbage Flea beetles, Phyllotreta cruciferae (Col. Chrys.). Ann Entomol Soc of Am 67:261–264
Higgins LG, Riley ND (1975) A field guide to the butterflies of Britain and Europe, 3rd ed. Collins, London
Hovanitz W (1963) The relation of Pieris virginiensis Edw. to P. napi L. J Res Lepidoptera 1:124–135
Jaenike J (1978) On optimal oviposition behaviour in phytophagous insects. Theor Popul Biol 14:350–356
Jones RE (1977) Movement patterns and egg distributions in cabbage butterflies. J Anim Ecol 46:195–212
Kjaer A (1976) Glucosinolates in the Cruciferae. In: Vaughan JG, Mac Leod AJ, Jones BMG (eds) The biology and chemistry of the cruciferae. Academic Press, London
Levins R, MacArthur RH (1969) An hypothesis to explain the incidence of monophagy. Ecology 50:910–911
Lewontin RC (1977) Fitness, survival and optimality. In: Horn, Mitchell, Stairs (eds) Analysis of ecological systems. Ohio State University Press
Lorkovic Z (1968) Systematisch — genetische und ökologische Besonderheiten von Pieris ergane Hbn. (Lep. Pier.). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 41:233–244
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Maynard Smith J (1978) Optimization Theory in evolution. Annu Rev Ecol System 9:35–56
Nair KSS, McEwen FL, Alex JF (1973) Oviposition and development of Hylemya brassicae Bouche on cruciferous weeds. Proc Entomol Soc Ontario 104:11–15
Nelson JB (1964) Factors influencing clutch size and chick growth in the North Atlantic Gannet (Sula bassana). Ibis 166:63–77
Newman E (1869) An illustrated natural history of British butterflies and moths. W.H. Allen, London
Nielsen JK (1978) Hostplant discrimination within Cruciferae: feeding responses of four leaf beetles (Col. Chrys.) to glucosinolates, curcubitains and cardenolides. Entomologia Exp Applicata 24:41–54
Ohsaki N (1979) Comparative population studies on three Pieris butterflies, P. rapae, P. melete and P. napi living in the same area. 1. Res Popul Ecol 20:278–296
Petersen B (1954) Egg-laying and habitat selection in some Pieris species. Entomologisk Tidskrift 75:194–203
Pulliam HR (1974) On the theory or optimal diets. Am Nat 108:59–74
Rausher MD (1978) Search image for leaf shape in a butterfly. Science 200:1071–1073
Rausher MD (1979) Larval habitat suitability and oviposition preference in 3 related butterflies. Ecology 60:503–511
Rodman J, Chew FS (1980) Phytochemical correlates of herbivory in a community of native and naturalized Cruciferae. Biochem System Ecol 8:43–50
Scriber JM (1973) Latitudinal gradients in larval feeding specialization of the world Papilionidae (Lepidoptera). Psyche 80:355–373
Sehgal UK, Vjagir R (1977) Plants natural defences in Cruciferae and Tropaeolaceae against mustard sawfly Athalia proxima Klug. Indian J Ecol 4:199–205
Shapiro AM (1971) Occurrence of a latent polyphenism in Pieris virginiensis (Lep. Pier.). Entomol News 82:13–16
Shapiro AM, Carde R (1970) Habitat selection and Competition among sibling species of satyrid butterflies. Evolution 24:48–54
Sih A (1979) Optimal diet: the relative importance of the different parameters. Am Nat 113:460–463
Singer MC (1971) Evolution of foodplant preference in the butterfly Euphydryas editha. Evolution 25:383–389
Smiley J (1978) Plant chemistry and the evolution of host specificity: new evidence from Heliconius and Passiflora. Science 201:745–747
Stanton ML (1979) The role of chemotactile stimuli in the oviposition preference of Colias butterflies. Oecologia 39:79–91
Takata N (1961) Studies on the host preference of common cabbage butterfly. XII. Japanese J Ecol 11:147–154
Varga Z (1967) Population studien über Pieris bryoniae O. in Karpathen becken. Acta Biol Debrecina 5:139–150
White RR, Singer MC (1974) Geographical distribution of hostplant choice in Euphydryas editha (Nymph). J Lepidopterists Soc 28:103–107
Wiklund C (1973) Hostplant suitability and the mechanism of host selection in larvae of Papilio machaon L. Entomol Exp Applicata 16:232–242
Wiklund C (1974a) The concept of oligophagy and the natural habitats and host plants of Papilio machaon L. Entomol Scand 5:151–160
Wiklund C (1974b) Oviposition preference in Papilio machaon in relation to the hostplants of the larvae. Entomol Exp Applicata 17:189–198
Wiklund C (1975) The evolutionary relationship between adult oviposition preferences and larval host plant range in Papilio machaon L. Oecologia 18:185–197
Wiklund C, Ahrberg C (1978) Hostplants, nectar source plants and habitat selection of males and females of Anthocharis cardamines. Oikos 31:169–183
Young AM (1978) Spatial properties of niche separation among Eueides and Dryas butterflies in Costa Rica. J New York Entomol Soc 86:2–19
Zwolfer H (1974) Speciation and ecological differentiation in phytophagous insects. Dtsch Zool Ges 67:394–401
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Courtney, S.P. Coevolution of pierid butterflies and their cruciferous foodplants. Oecologia 54, 101–107 (1982). https://doi.org/10.1007/BF00541116
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00541116