Abstract
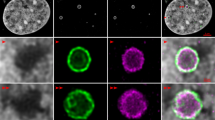
The coiled body is a phylogenetically conserved nuclear organelle whose function is not known. Probes for detection of p80-coilin, an 80 kDa protein enriched in the coiled body, have made possible studies determining the behavior of the coiled body during the cell cycle, in proliferating cells, as well as reports suggesting some relationship of the coiled body to mRNA splicing and to the nucleolus. The objective of this study is to examine the distribution of p80-coilin and nucleolar proteins in cells infected with adenovirus in vitro. HeLa cells grown as monolayers were infected with successive dilutions of type 5 human adenovirus culture and fixed in methanol/acetone at different time points. Single and double indirect immunofluorescence was performed with human autoantibodies to p80-coilin, fibrillarin, NOR-90/hUBF, RNA polymerase I, PM-Scl, and To, as well as rabbit polyclonal serum to p80-coilin (R288) and mouse monoclonal antibody to adenovirus 72-kDa DNA-binding protein. Indirect immunofluorescence (IIF) with anti- p80-coilin antibodies showed that the usual bright dot-like coiled body staining pattern was replaced in infected cells by 1–5 clusters of tiny dots at the periphery of the nucleus. This phenomenon was first detected within 12 h of infection and affected more severely cells with increased length and load of infection. Cells subjected to heat shock presented no such alteration. Double IIF showed that cells with abnormal coiled body appearance expressed the viral 72-kDa DNA-binding protein. Nucleolar proteins RNA polymerase I and NOR-90/hUBF became associated with the p80-coilin-enriched clusters and were no longer detected in the nucleolus. Other nucleolar proteins, like PM-Scl and To, remained associated to the nucleolus and were not detected in the newly formed clusters. Fibrillarin had a heterogeneous behavior, being restricted to the nucleolus in some infected cells while in some others it was associated with the p80-coilin-enriched clusters. Thus our results showed that in vitro adenovirus infection induced radical redistribution of nucleolar and coiled body constituents into newly formed structures characterized by clusters of tiny dots in the periphery of the nucleus. The fact that three major proteins involved in rRNA synthesis and processing colocalized with p80-coilin in these clusters may bring additional support to the idea that the coiled body and p80-coilin may be implicated in functions related to the nucleolus.
Similar content being viewed by others
References
Ramon Y Cajal S (1903) Trab. Lab. Invest. Biol. 2: 129–221
Hardin JH, Spicer SS & Greene WB (1969) Anat. Rec. 164: 403–432
Seite R, Pebusque M-J & Vio-Cigna M (1982) Biol. Cell. 46: 97–100
Lafarga M, Hervas JP, Santa-Cruz MC, Villegas J & Crespo D (1983) Anat. Embryol. 166–175
Andrade LEC, Chan EKL, Raska I, Peebles CL, Roos G & Tan EM (1991) J. Exp. Med. 173: 1407–1419
Andrade LEC, Tan EM & Chan EKL (1993) Proc Natl. Acad. Sci USA 90: 1947–1951
Carmo-Fonseca M, Ferrira J & Lamond A (1993) J. Cell. Biol. 120: 841–852
Ochs RL, Stein TWJr., Andrade LEC, Gallo D, Chan EKL, Tan EM & Brasch K (1995) Mol. Biol. Cell. 6: 345–356
Raska I, Andrade LEC, Ochs RL, Chan EKL, Chang C-M, Roos G & Tan EM (1991) Exp. Cell. Res. 195: 27–37
Frey MR & Matera G (1995) Proc. Natl. Acad. Sci. USA 92: 5915–5919, 1995
Fakan S, Leser G & Martin TE (1984) J. Cell. Biol. 98: 358–363
Carmo-Fonseca M, Pepperkok R, Carvalho MT & Lamond A (1992) J. Cell. Biol. 117: 1–14
Matera AG & Ward DC (1993) J. Cell. Biol. 121: 715–727
Raska I, Ochs RL, Andrade LEC, Chan EKL, Burlingame R, Peebles CP, Gruol D & Tan EM (1990) J. Struct. Biol. 104: 120–127
Bohmann K, Ferreira J, Santama N, Weis K & Lamond AI (1995) J. Cell. Sci. (Suppl. 19) 107–113
Ochs RL, Stein TWJr. & Tan EM (1994) J. Cell. Sci. 107: 385–399, 1994
Malatesta M, Zancanaro C, Martin TE, Chan EKL, Almaric F, Lührmann R, Vogel P & Fakan S (1994) Eur. J. Cell. Biol. 65: 82–93
Meier UT & Blobel G (1992) Cell 70: 127–138
Meier UT & Blobel G (1994) J. Cell. Biol. 127: 1505–1514
Jimenéz-Garcia LF, Segura-Valdez M de L, Ochs RL, Rothblum L, Hannan R & Spector DL (1994) Mol. Biol. Cell. 5: 955–966
Bohmann K, Ferreira JA & Lamond A (1995) J. Cell. Biol. 131: 817–831
Puvion-Dutilleul F, Bachellerie JP, Visa N & Puvion E (1994) J. Cell. Sci. 107: 1457–1468
Bridge E, Xia D-X, Carmo-Fonseca M, Cardinali B, Lamond AI & Pettersson U (1995) J. Virol. 69: 281–290
Puvion-Dutilleul F & Christensen ME (1993) Eur. J. Cell. Biol. 61: 168–176
Branton PE, Evelegh M, Rowe DT, Graham FL & Bacchetti S (1985) Can. J. Biochem. Cell. Biol. 63: 941–952
Reimer G, Pollard KM, Penning CA, Ochs RL, Lischwe MA, Busch H & Tan EM (1987) Arthritis Rheum. 30: 793–800
Reddy R, Tan EM, Henning D, Nohga K & Busch H (1983) J. Biol. Chem. 258: 1381–1386
Reichlin M, Maddison PJ, Targoff IN, Bunch T, Arnett F, Sharp G, Treadwell E & Tan EM (1984) J. Clin. Invest. 4: 40–45
Chan EKL, Imai H Hamel JC & Tan EM (1991) J. Exp. Med. 174: 1239–1244
Reimer G, Rose KM, Scheer U & Tan EM (1987) J. Clin. Invest. 79: 65–72
Horwitz MS: Adenoviridae & their replication. In: Fields BN & Knipe DM, eds. Virology, Second Edition, Raven Press Ltd., New York, 1990, pp. 1679–1712
Beltz GA & Flint SJ (1979) J. Mol. Biol. 131: 353–373
Raskas HJ, Thomas DC & Green M (1970) Virology 40: 893–899, 1970
Rebelo L, Almeida F, Ramos C, Bohmann K, Lamond AI & Carmo-Fonseca M (1996) Mol. Biol. Cell. 7: 1137–1151
Benavente R, Reimer G, Rose KM, Hügle-Dörr B & Scheer U (1988) Chromosoma (Berlin) 97: 115–123
Wagner EK & Roizman B (1969) J. Virol. 4: 36–46
Yokoyama Y, Niwa K & Tamaya T (1992) Exp. Cell. Res. 202: 77–86
Lamond A & Carmo-Fonseca M (1993) Trends Cell. Biol. 3: 198–204
Wu Z, Murphy C, Wu C-HH, Tsvetkov A & Gall JG (1993) CSH Symp. Quantit. Biol. 58: 747–754
Hervas JP, Villegas J, Crespo D & Lafarga M (1980) Am. J. Anat. 159: 447–454
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodrigues, S.H., Silva, N.P., Delício, L.R. et al. The behavior of the coiled body in cells infected with adenovirus in vitro . Molecular Biology Reports 23, 183–189 (1996). https://doi.org/10.1007/BF00351167
Issue Date:
DOI: https://doi.org/10.1007/BF00351167