Abstract
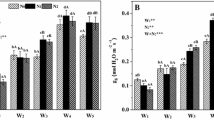
In the cellulose of stems and leaves, δ13C was investigated in a birch clone (Betula pendula), which was exposed throughout the growing season to either <3 (control) or 90/40 nl O3 1-1 (day/night). Each regime was split into plants under high or low nutrient supply. δ13C was increased (becoming less negative), in stems rather than leaves, by both high nutrition (+2‰) and O3 stress (+1‰). Whereas high nutrition raised the wateruse efficiency (WUE) while lowering the CO2 concentration in the inner leaf air space (c i), WUE decreased and c i increased under O3 stress. Therefore, only the nutritional effect on the carbon isotope fractionation was reproduced by the model of Farquhar et al. (1982) which estimates WUE by means of δ13C based on c i. c i was not biased by ‘patchiness’ in respect to stomatal opening. The latter was verified by microscopical analysis and the complete water infiltration of the birch leaves through the stomata, independent of the diurnal course of the leaf conductance for water vapour. Under low nutrient supply, the activity of phosphoenol pyruvate carboxylase (PEPC) was roughly doubled by ozone to about 1.3% of the total carboxylation capacity (by PEPC + rubisco), and was increased to 1.7% under high nutrition. The fractionation model, extended to account for varying activities of the carboxylating enzymes, indicated that stimulated PEPC was the cause of elevated δ13C, although c i was increased under O3 stress. The stimulation of PEPC and, as a consequence, elevated δ13C are discussed as part of a whole-plant acclimation to O3 stress.
Similar content being viewed by others
References
Beyschlag W, Pfanz H (1990) A fast method to detect the occurrence of nonhomogeneous distribution of stomatal aperture in heterobaric plant leaves. Experiments with Arbutus unedo L. during the diurnal course. Oecologia 82:52–55
Beyschlag W, Wedler M, Lange OL, Heber U (1987) Einfluß einer Magnesiumdüngung auf Photosynthese und Transpiration von Fichten an einem Magnesium-Mangelstandort im Fichtélgebirge. Allg. Forst Z. 42:738–741
Björkman O (1981) Response to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology. Physiological plant ecology. I. Response to physical environment. (New series, vol 12A) Springer, Berlin Heidelberg New York, pp 57–107
Boutton TW, Flagler RB (1991) 13C/12C ratios as indicators of plant physiological response to elevated ozone and simulated acid rain. In: Stable isotopes in plant nutrition, soil fertility and environmental studies, (Proceedings series) IAEA, Vienna, pp 627–631
Cheeseman JM (1991) PATCHY: simulating and visualizing the effects of stomatal patchiness on photosynthetic CO2 exchange studies. Plant Cell Environ 14: 593–599
Condon AG, Richards RA, Farquhar GD, (1992) The effect of variation in soil water availability, vapour pressure deficit and nitrogen nutrition on carbon isotope discrimination in wheat. Aust J Agric Res 43:935–947
Craig H (1957) Isotopic standards for carbon and oxygen and cor rection factors for mass spectrometric analyses of carbon dioxide. Geochim Cosmochim Acta 12: 133–149
Dann MS, Pell EJ (1989) Decline of activity and quantity of ribu lose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiol 91: 427–432
Deines P (1980) The isotopic composition of reduced organic carbon. In: Fritz P, Fontes JC (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, pp 329–406
Ehleringer JR, Phillips SL, Comstock JP (1992) Seasonal variation in the carbon isotopic composition of desert plants. Funct Ecol 6: 396–404
Elsik CG, Flagler RB, Boutton TW (1993) Carbon isotope composition and gas exchange of loblolly and shortleaf pine as affected by ozone and water stress. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon-water relations. Academic Press, San Diego, pp 227–244
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11: 539–552
Farquhar GD, O'Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9: 121–137
Farquhar GD, Ehleringer JR, Hubick KT (1989a) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537
Farquhar GD, Hubick KT, Condon AG, Richards RA (1989b) Carbon isotope fractionation and plant water-use efficiency. In: Stable isotopes in ecological research. Springer, Berlin Heidelberg New York, pp 21–40
Freyer HD (1979) On the δ13C record in tre rings. Part II. Registration of microenvironmental CO2 and anomalous pollution effect. Tellus 31: 308–312
Greitner CS, Winner WE (1988) Increases in δ13C values of radish and soybean plants caused by ozone. New Phytol 108: 489–494
Günthardt-Goerg MS, Matyssek R, Scheidegger C, Keller T (1993) Differentiation and structural decline in the leaves and bark of birch (Betula pendula) under low ozone concentration. Trees 7: 104–114
Hickey LJ, (1973) Classification of the architecture of dicotyledonous leaves. Am J Bot 60: 17–33
Holbrook GP, Keys AJ, Leech RM (1984) Biochemistry of photosynthesis in species of Triticum of different ploidy. Plant Physiol 74: 12–15
Hubick KT (1990) Effects of nitrogen source and water limitation on growth, transpiration efficiency and carbon-isotope discrimination in peanut cultivars. Aust J Plant Physiol 17: 413–430
Ingestad T, Lund A-B (1986) Theory and techniques for steadystate mineral nutrition and growth of plants. Scand J For Res 1: 439–453
Küppers M, Zech W, Schulze E-D, Beck E (1985) CO2-Assimilation, Transpiration und Wachstum von Pinus sylvestris L. bei unterschiedlicher Magnesiumversorgung. Forstw Cbl 104: 23–36
Landolt W, Pfenninger I, Lüthy-Krause B (1989) The effect of ozone and season on the pool sizes of cyclitols in Scots pine (Pinus sylvestris) Trees 3: 85–88
Landolt W, Günthardt-Georg MS, Pfenninger I, Scheidegger CH (1994) Ozone-induced microscopical changes and quantitative carbohydrate contents of hybrid poplar (Populusxeuramericana). Trees 8: 183–190
Lauer MJ, Boyer JS (1992) Internal CO2 measured directly in leaves, abscisic acid and low leaf water potential cause opposing effects. Plant Physiol 98: 1310–1316
Luethy-Krause B, Pfenninger I, Landolt W (1990) Effects of ozone on organic acids in needles of Norway spruce and Scots pine. Trees 4: 198–204
Martin B, Bytherowicz A, Thorstenson R (1988) Effects of air pollutants on the composition of stable carbon isotopes, δ13C, of leaves and wood, and on leaf injury. Plant Physiol 88: 218–223
Matyssek R, Günthardt-Georg MS, Keller TH, Scheidegger CH (1991) Impairment of gas exchange and structure in birch leaves (Betula pendula) caused by low ozone concentrations. Trees 5: 5–13
Matyssek R, Günthardt-Georg MS, Saurer M, Keller T (1992) Seasonal growth, δ13C in leaves and stem, and phloem structure of birch (Betula pendula) under low ozone concentrations. Trees 6: 69–76
Matyssek R, Günthardt-Goerg MS, Landolt W, Keller T (1993) Whole-plant growth and leaf formation in ozonated hybrid poplar (Populusxeuramericana). Environ Pollut 81: 207–212
Matyssek R, Günthardt-Goerg MS, Maurer S, Keller T (1995) Nighttime exposure to ozone reduces whole-plant production in Betula pendula. Tree Physiol 15: 159–165
Mooney HA, Winner WE (1991) Partitioning response of plants to stress. In: Mooney HA, Winner WE, Pell EJ (eds) Response of plants to multiple stresses. Academic Press, San Diego, pp 129–141
Neger FW (1918) Die Wegsamkeit der Laubblätter für Gase. Flora 3: 152–161
Polle A (1995) Protection from oxidative stress in trees as affected by elevated CO2 and environmental stress. In: Mooney HA, Koch GW (eds) Terrestrial ecosystem response to elevated CO2. (Physiological ecology series) Academic Press, New York London (in press)
Reich PB (1983) Effects of low concentrations of O3 on net photosynthesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol 73: 291–296
Reich PB, Amundson RG (1985) Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science 230: 566–570
Saurer M, Fuhrer J, Siegenthaler U (1991) Influence of ozone on the stable carbon isotope composition, δ13C, of leaves and grain of spring wheat (Triticum aestivum L). Plant Physiol 97: 313–316
Scheidegger C, Günthardt-Goerg MS, Matyssek R, Hatvani P (1991) Low-temperature scanning electron microscopy of birch leaves after exposure to ozone. J Microsc 161: 85–95
Schmidt G, Gebauer G, Widmann K, Ziegler H (1993) Influence of nitrogen supply and temperature on stable carbon isotope ratios in plants of different photosynthetic pathways (C3, C4, CAM). Isotopenpraxis Environ Health Stud 29: 9–13
Schmieden-Kompalla U, Hartmann U, Korthals S, Wild A (1989) Activity and activation state of ribulose-1,5-bisphosphate carboxylase of spruce trees with varying degree of damage relative to the occurrence of novel forest decline. Photosynth Res 21: 161–169
Schulze E-D (1986) Carbon dioxide and water vapor exchange in response to drought in the atmosphere and in the soil. Annu Rev Plant Physiol 37: 247–274
Terashima I, Wong S-C, Osmond CB, Farquhar GD (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol 29: 385–394
Troughton JH, Card KA, Hendy CH (1974) Photosynthetic pathways and carbon isotope discrimination by plants. Carnegie Inst Wash Yearbook 73: 768–780
Winner WE, Gillespie C, Shen W-S, MooneyHA (1988) Stomatal responses to SO2 and O3. In: Schulte-Hostede S, Darral NM, Blank LW, Wellburn AR (eds) Air pollution and plant metabolism. Elsevier, London, New York pp 255–271
Winter K (1981) CO2 and water vapour exchange, malate content and δ13C value in Cicer arietinum grown under two water regimes. Z Pflanzenphysiol 101: 421–430
Wiskich JT, Dry IB (1985) The tricarboxylic acid cycle in plant mitochondria: its operation and regulation. In: Douce R, Day DA (eds) Higher plant cell respiration. Encyclopaedia of Plant Physiology. (New series, vol 18) Springer, Berlin Heidelberg New York, pp 281–313
Wolfenden J, Mansfield TA (1991) Physiological disturbances in plants caused by air pollutants. Proc R Soc Edinburgh 97B: 117–138
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saurer, M., Maurer, S., Matyssek, R. et al. The influence of ozone and nutrition on δ13C in Betula pendula . Oecologia 103, 397–406 (1995). https://doi.org/10.1007/BF00328677
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328677