Summary
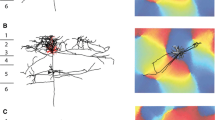
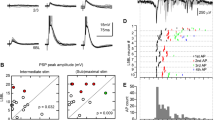
Responses evoked in neurons of rat sensorimotor cortex upon stimulation of the pyramidal tract and ipsilateral cerebral peduncle were analysed using intracellular recording. Neurons responding antidromically to pyramidal tract stimulation (PT cells) and neurons failing to respond anti-dromically but exhibiting orthodromic responses were both stained by intracellular injection of horse-radish peroxidase (HRP). Layer V pyramidal neurons, including those responding antidromically, exhibited prominent long lasting membrane hyperpolarizations and inhibitions of action potentials following pyramidal tract or cerebral peduncle stimulation. Upon passage of polarizing intracellular current two components were identified within the hyperpolarizing potential. A short duration initial component readily reversed with hyperpolarizing current. Frequently this earlier component over-lapped a period of early excitation consisting of action potentials arising from recurrent EPSPs or large slow depolarizing potentials (SDPs). The second, much longer duration hyperpolarizing component did not reverse with passage of hyperpolarizing current and was often followed by a rebound period of depolarization and action potential generation. Both the excitatory and the inhibitory portions of these responses could be demonstrated in animals with acute thalamic transections severing the ascending lemniscal pathway to cortex. Following intracellular staining with HRP, two types of PT cells were identified by their different intracortical axonal arborizations. Most of the injected neurons had local axonal fields extending widely in layers V and VI, but with few or no collaterals extending radially toward the more superficial layers. A second type of PT cell had axon collaterals limited to a narrow zone around the dendritic field but extending radially as far as layer I. Cells of both types were observed to send axon collaterals into neostriatum. Both types of neurons exhibited morphological and physiological characteristics of slow PT cells, and we could find no cells comparable to the fast conducting PT cells observed in other species.
Similar content being viewed by others
References
Brown LT (1971) Projections and terminations of the corticospinal tract in rodents. Exp Brain Res 13: 432–451
Catsman-Berrovoets CE, Lemon RN, Verberg CA, Bentivoglio M, Kuypers HGJM (1980) Absence of callosal collaterals derived from rat corticospinal neurons. A study using fluorescent retrograde tracing and electrophysiological techniques. Exp Brain Res 39: 433–440
Connors BW, Gutnik MJ, Prince DA (1982) Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol 48: 1302–1320
Deschênes M, Labelle A, Landry P (1979a) A comparative study of ventrolateral and recurrent excitatory postsynaptic potentials in large pyramidal tract cells in the cat. Brain Res 160: 37–46
Deschênes M, Labelle A, Landry P (1979b) Morphological characterization of slow and fast pyramidal tract cells in the cat. Brain Res 178: 251–274
Deschênes M, Roy JP, Steriade M (1982) Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res 239: 289–293
Donoghue JP, Kitai ST (1981) A collateral pathway to neostriatum from neurons in somatic sensory-motor cortex demonstrated by intracellular labeling with HRP. J Comp Neurol 201: 1–13
Donogue JP, Wise SP (1982) The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol 212: 76–88
Dutar P, Lamour Y, Jobert A (1982) Differences in physiological and pharmacological properties of 3 types of pyramidal neurons identified by antidromic stimulation in the first somatic sensory cortex of the rat. CR Seances Acad Sci Ser III Sci Vie 294: 711–714
Endo KT, Araki T, Yagi N (1973) The distribution and patterns of axon branching of pyramidal tract cells. Brain Res 57: 484–491
Evarts EV (1965) Relation of discharge frequency to conduction velocity in pyramidal tract neurons. J Neurophysiol 28: 216–228
Hamada I, Sakai M, Kubota K (1981) Morphological differences between fast and slow pyramidal tract neurons in the monkey motor cortex as revealed by intracellular injection of horse-radish peroxidase by pressure. Neurosci Lett 22: 233–238
Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res 175: 341–346
Jinnai K, Matsuda Y (1979) Neurons of the motor cortex projecting commonly on the caudate nucleus and the lower brain stem in the cat. Neurosci Lett 13: 121–126
Kennedy TT, Towe AL (1962) Identification of a fast lemniscocortical system in the cat. J Physiol (Lond) 160: 535–547
Kitai ST, Kocsis JD, Wood J (1976) Origin and characteristics of the cortico-caudate afferents: an anatomical and electro-physiological study. Brain Res 118: 137–141
Labelle A, Deschênes M (1979) Differential distribution of spines on the apical dendrites of slow and fast pyramidal tract cells in the cat. Brain Res 164: 309–313
Landry P, Labelle A, Deschênes M (1980) Intracortical distribution of axon collaterals of pyramidal tract cells in the cat motor cortex. Brain Res 191: 327–336
Llinás R, Jahnsen H (1982) Electrophysiology of mammalian thalamic neurones in vitro. Nature 297: 406–408
McComas AJ, Wilson P (1968) An investigation of pyramidal tract cells in the somatosensory cortex of the rat. J Physiol (Lond) 194: 271–288
Mediratta NK, Nicoll JAR (1983) Conduction velocities of corticospinal axons in the rat studied by recording cortical antidromic responses. J Physiol (Lond) 336: 545–561
Miller R (1975) Distribution and properties of commissural and other neurons in cat sensorimotor cortex. J Comp Neurol 164: 361–374
Ohata M (1968) Cortical motor function and the antidromic cortical response to stimulation of the medullary pyramidal tract in the rat. Jpn J Physiol 18: 100–124
Oka H, Jinnai K (1978) Common projection of the motor cortex to the caudate nucleus and the cerebellum. Exp Brain Res 31: 31–42
Porter R (1981) Internal organization of the motor cortex for input-output arrangements. In: Brooks VB (ed) Handbook of physiology, Vol II. Am Physiol Soc, Baltimore, pp 1063–1081
Porter R, Sanderson JH (1964) Antidromic cortical response to pyramidal-tract stimulation in the rat. J Physiol (Lond) 170: 355–370
Prince DA (1968) The depolarization shift in “epileptic” neurons. Exp Neurol 21: 467–485
Scheibel ME, Scheibel AB (1978) The dendritic structure of the human Betz cell. In: Brazier MAB, Petsche H (eds) Architectonics of the cerebral cortex. Raven Press, New York, pp 43–57
Stefanis C, Jasper H (1964a) Intracellular microelectrode studies of antidromic responses in cortical pyramidal tract neurons. J Neurophysiol 27: 828–854
Stefanis C, Jasper H (1964b) Recurrent collateral inhibition in pyramidal tract neurons. J Neurophysiol 27: 855–877
Stone TW (1972) Cortical responses to pyramidal tract stimulation in the rat. Exp Neurol 35: 492–502
Takahashi K (1965) Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol 28: 908–924
Takahashi K, Kubota K, Uno M (1967) Recurrent facilitation in cat pyramidal tract cells. J Neurophysiol 30: 22–34
Tsukahara N, Fuller DRG, Brooks VB (1968) Collateral pyramidal influences on the corticorubrospinal system. J Neurophysiol 31: 467–484
Wilson CJ, Chang HT, Kitai ST (1982) Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp Brain Res 45: 157–167
Wilson CJ, Chang HT, Kitai ST (1983) Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res 51: 227–235
Wilson CJ, Groves PM (1979) A simple and rapid section embedding technique for sequential light and electron microscopic examination of individually-stained central neurons. J Neurosci Meth 1: 383–391
Wise SP, Jones EG (1977) Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol 175: 129–158
Yelnik J, Percheron C (1979) Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience 4: 1717–1743
Author information
Authors and Affiliations
Additional information
Supported by NIH grants NS 20743 (to CJW), NS 20702 (to STK) and a FRSQ fellowship (to PL)
Rights and permissions
About this article
Cite this article
Landry, P., Wilson, C.J. & Kitai, S.T. Morphological and electrophysiological characteristics of pyramidal tract neurons in the rat. Exp Brain Res 57, 177–190 (1984). https://doi.org/10.1007/BF00231144
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231144