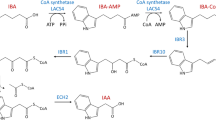
Abstract
We have studied the effect of a change in the endogenous hormone equilibria on the competence of tomato (Lycopersicon esculentum) cells to defend themselves against the fungal pathogen Fusarium oxysporum f. sp. lycopersici. Calluses from cvs ‘Davis’ and ‘Red River’, respectively resistant and susceptible to Fusarium and transgenic for an auxin- or cytokinin-synthesizing gene from Agrobacterium tumefaciens, were used. The integration of Agrobacterium hormone-related genes into susceptible cv ‘Red River’ can bring the activation of defense processes to a stable competence as assessed by the inhibition of mycelial growth in dual culture and gem-tube elongation of Fusarium conidia, the determination of callose contents, peroxidase induction and ion leakage in the presence of fusaric acid. This is particularly true when the transformation results in a change of phytohormone equilibria towards an higher cytokin in concentration. On the contrary, in resistant cv ‘Davis’ the inhibition of both fungal growth in dual culture and conidia germination is higher when the hormone balance is modified in favour of the auxins. No significant effect was observed for ion leakage and peroxidase induction, probably because of a constitutive overproduction of cytokinins in ‘Davis’ cells.
Similar content being viewed by others
References
Balazs E, Sziraki I, Kiraly Z (1977) The role of cytokinins in the systemic acquired resistance of tobacco hypersensitive to tobacco mosaic virus. Physiol Plant Pathol 11:29–37
Beckman CH (1987) The nature of wilt disease of plants. APS Press, St. Paul, Minnesota
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein-dye binding. Anal Biochem 72:248–254
Buiatti M, Scala A, Bettini P, Nascari G, Morpurgo R, Bogani P, Pellegrini MG, Gimelli F, Venturo R (1985) Correlation between in vivo resistance to Fusarium and in vitro response to fungal elicitors and toxic substances in carnation. Theor Appl Genet 70:42–47
Buiatti M, Simeti C, Marcheschi G, Scala A, Bettini P, Bogani P, Pellegrini MG (1987) Isolation of tomato cell lines with altered response to Fusarium cell wall components. Theor Appl Genet 75:37–40
Corsini DL, Thompson C, Pavek JJ (1989) The effects of plant growth regulators on Verticillium wilt of potato. Am Potato J 66:125–136
Dua IS, Suman BC, Rao AV (1978) Resistance of cauliflower (Brassica oleracea var ‘botrytis’) to Xanthomonas campestris (Pam) Dowson as influenced by endogenous growth substances and relative growth rate. Indian J Exp Biol 16:488–491
Eidel'nant NM, Esipova IV, Demurina AK, Kryukova LI (1983) Content of cytokinins and abscissic in healthy wheat plants and those infected with Puccinia striiformis. Uzb Biol Zurnali 3:26–27
Faccioli G, Rubies-Autonell C, Albertini R (1984) Role of cytokinins in the acquired resistence of Chenopodium amaranticolor towards an infection of tobacco necrosis virus. Phytopathol Medit 23:15–22
Fedorova AI, Gagulaeva AP (1976) Relationship between resistance to Pristiphora wesmaeli in Larix sibirica and its growth rate and content of phenols. Izv Sib Otd Akad Nauk SSSR, Ser Biol 2:80–85
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Feinberg AP, Vogelstein B (1984) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Addendum. Anal Biochem 137:266–267
Flor HH (1942) Inheritance of pathogenicity in Melampsora lini. Phytopathology 32:653–669
Goossens JFV, Vendring JC (1982). Effects of abscissic acid, cytokinins and light on isoflavonoid phytoalexin accumulation in Phaseolus vulgaris L. Planta 154:441–446
Haskins F (1955) Changes in the activities of several enzymes during germination and seedling development in corn (Zea mays L.) Plant Physiol 30:74–79
Hirsch PR, Beggs JD (1984) Agrobacterium tumefaciens T-DNA in the yeast Saccharomyces cerevisiae. Mol Gen Genet 195:209–214
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whister RL, Wolfram ML (eds) Methods in carbohydrate chemistry. Academic Press, New York, pp 380–394
Horsch RB, Fry J, Hoffmann NL, Wallroth M, Eicholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Inzé D, Follin A, Van Lijsebettens M, Simoens C, Genetello C, Van Montagu M, Schell J (1984) Genetic analysis of the individual T-DNA genes of Agrobacterium tumefaciens; further evidence that two genes are involved in indole-3-acetic acid synthesis. Mol Gen Genet 194:265–274
Kaplan IB, Malyshenko SI, Shakulova ER, Dubinina EN, Tal'yanskii ME (1988) Induction of pathogenesis-related proteins and acquired antiviral resistance in tobacco plants under the influence of kinetin. Sov Plant Physiol 35:650–655
Kay LE, Basile DV (1987) Specific peroxidase isoenzymes are correlated with organogenesis. Plant Physiol 84:99–105
Kirn YH, Chae YA (1985) Selection of resistant cell lines to Phytophthora parasitica var ‘nicotianae’ at callus level and plant regeneration in Nicotiana tabacum. Agric Res Seoul Natl Univ 10:29–34
Kohle H, Jeblick W, Polen F, Blaschek W, Kauss H (1985) Chitosanelicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol 77:544–551
Kratka J, Kudela V (1981) Biochemical changes in alfalfa plants inoculated with Verticillium albo-atrum. Phytopathol Z 100:289–299
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissues cultures. Plant Physiol 18:100–127
Madamanchi NR, Kuć J (1991) Induced systemic resistance in plants. In: Cole GT, Hoch TA (eds) Fungal spore and disease initiation in plants and animals. Plenum Publ, New York, pp 347–362
Matsumoto K, Suzukia Y, Mase S, Watanabe I, Sekizawa Y (1980) On the relationship between plant hormones and rice blast resistance. Ann Phytopathol Soc Jpn 46:307–314
Memelink J, Hoge JHC, Schilperoort RA (1987) Cytokinin stress changes the developmental regulation of several defense-related genes in tobacco. EMBO J 6:3579–3583
Memelink J, Linthorst JM, Schilperoot RA, Hoge HC (1990) Tobacco genes encoding acidic and basic isoforms of pathogenesis-related proteins display different expression patterns, Plant Mol Biol 14:119–126
Mills PR, Gussine J, Wood RSK (1986) Induction of resistance in cucumber to Colletotrichum lagenarium by 6-benzylaminopurine. J Phytopathol 116:11–17
Mohnen D, Shinshi H, Felix G, Meins F Jr (1985) Hormonal regulation of β-1,3 glucanase messenger RNA levels in cultured tobacco tissues. EMBO J 4:1631–1635
Plich M (1976) Influence of growth regulators on the development of collar knot disease caused by the fungus Phytophthora cactorum in apple trees. Fruit Sci Rep 3:33–42
Reed KC, Mann DA (1985) Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acid Res 13:7207–7221
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Sarhan ART, Kirialy Z, Sziraki I, Smedegaard-Petersen V (1991) Increased levels of cytokinins in barley leaves having the systemic acquired resistance to Bipolaris sorokiniana (Sacc.). J Phytopathol 131:101–108
Scala A, Bettini P, Buiatti M, Collina Grenci F, Pellegrini MG, Bogani P, Lipucci di Paola M, Tognoni F (1983) Induction of antimicrobial compounds in tomato callus by cell wall components from Phytophthora infestans Mont. de Bary. Phytopathol Medit 22:143–146
Shinshi H, Mohnen D, Meins F Jr (1987) Regulation of a plant pathogenesis-related enzyme: inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci USA 84:89–93
Storti E, Bogani P, Bettini P, Bonzi Morassi L, Pellegrini MG, Simeti C, Buiatti M (1989) The pleiotropic phenotype of tomato cells selected for altered response to Fusarium oxysporum f. sp. lycopersici cell-wall components. Theor Appl Genet 78:689–695
Storti E, Buiatti M, Bettini P, Pellegrini MG, Bogani P, Matteo M, Simeti C (1990) Active defense processes and in vitro selection for resistance to pathogens. Acta Hortic 280:361–366
Storti E, Latil C, Salti S, Bettini P, Bogani P, Pellegrini MG, Simeti C, Molnar A, Buiatti M (1992) The in vitro physiological phenotype of tomato resistance to Fusarium oxysporum f. sp. lycopersici. Theor Appl Genet 84:123–128
Tuzun S, Kuc' J (1987) Persistence of induced systemic resistance to blue mold in tobacco plants derived via tissue culture. Phytopathology 77:1032–1035
Tuzun S, Kuc' J (1989) Induced systemic resistance to blue mold. In: McKeen WE (ed). Blue mold of tobacco. American Phytopathological Society Press, St. Paul, Minnesota, pp 177–200
Vizarova G (1985) Hypothetical role of free endogenous zeatin and its derivatives in the cereals' resistance to obligate fungus parasites. Acta Univ Agric Brno 33:175–180
Vizarova G (1987) Possible role of cytokinins in cereals regard to the resistance to obligate fungus parasites. Biol Plant 29:230–233
Vizarova G, Muzikova D (1981) The content of free endogenous cytokinins in the grain of barley and wheat in relation to their resistance to mildew. Pol'nohospordarstvo 27:1109–1115
Vizarova G, Vozar I (1984) Free endogenous cytokinin content in the seeds of barley (Hordeum sativum) and wheat (Triticum vulgare) cultivars with different resistance to powdery mildew (Erysiphe graminis). Biochem Physiol Pflanz 179:767–774
Vizarova G, Shashkova LS, Andreev LN (1988) On the question of the relationship between free zeatin content and resistance of wheat to biotrophic fungi. Acta Phytopathol Entomol Hung 23:385–392
Wieringa-Brans DH, Dekker WC (1987) Induced resistance in hypersensitive tobacco against tobacco mosaic virus by injection of intercellular fluid from tobacco plants with systemic-acquired resistance. J Phytopathol 118:165–170
Zakhar'ev M, Tashpulatov ASH, Nurkiyanova KM, Tal'yanskii ME, Kaplan IB, Atabekov IG, Skryabin KG (1988) Endogenous cytokinin-induced PR-protein synthesis in Nicotiana tabacum plants. Dokl Akad Nauk SSSR 301:743–745
Author information
Authors and Affiliations
Additional information
Communicated by G. Wenzel
Rights and permissions
About this article
Cite this article
Storti, E., Bogani, P., Bettini, P. et al. Modification of competence for in vitro response to Fusarium oxysporum in tomato cells. II. Effect of the integration of Agrobacterium tumefaciens genes for auxin and cytokinin synthesis. Theoret. Appl. Genetics 88, 89–96 (1994). https://doi.org/10.1007/BF00222399
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222399