Abstract
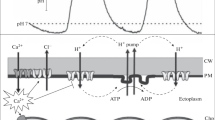
In caulonemal filaments of Physcomitrella patens which had been preincubated in the dark for 24 h, irradiation with red light (640 nm, fluence rate 85 μmol · m−2 · s−1) evoked (i) the development of side branch initials and (ii) a rapid, but transient, depolarisation of the plasma membrane by 90 ± 13 mV from a resting potential of -178 ± 13 mV. This was followed by a transient hyperpolarisation to a value 21± 8 mV more negative than the original membrane potential. The refractory period for the transient depolarisation was between 12 and 15 min. The fluence rate of red light required to evoke maximal depolarisation was about 80 μmol · m−2 · s−1 for a 1-min pulse. At this fluence rate, a depolarising response could be recorded for pulse lengths as small as 7 s. The transient depolarisation was insensitive to 3-(3′,4′dichlorophenyl)-1,1-dimethyl urea (DCMU) and was unchanged in plants bleached by growth on norflurazon (SAN 9789). Furthermore, the electrical response could be blocked by simultaneous application of far-red light. These results suggest the involvement of the photoreceptor phytochrome in the response. Removing Ca2+ from the external medium or replacing Ca2+ with Mg2+ blocked the depolarisation. The depolarisation could also be blocked by the K+ channel-blocker tetraethylammonium (10 mM) and the Cl− channel-blocker niflumic acid (1 μM). Conversely, although calcium channel-antagonists such as nifedipine and lanthanides, applied at a concentration of 100 μM, also altered the response, they did not block it. A possible ionic mechanism for the membrane potential transient is advanced, and the physiological significance discussed in the context of early events in the phytochrome signalling pathway.
Similar content being viewed by others
Abbreviations
- [Ca2+]c :
-
cytosolic Ca2+ concentration
- DCMU:
-
3-(3′,4′-dichlorophenyt)-1,1-dimethylurea
- TEA:
-
tetraethylammonium
References
Beilby MJ (1984) Calcium and plant action potentials. Plant Cell Environ 7: 415–421
Chae Q, Park HJ, Hong SD (1990) Loading of quin 2 into the oat protoplasts and measurement of cytosolic calcium ion concentration changes by phytochrome action. Biochim Biophys Acta 1051: 115–122
Cove DJ, Knight CD (1993) The moss Physcomitrella patens, a model system with potential for the study of plant reproduction. Plant Cell 5: 1483–1488
Cove DJ, Schild A, Ashton NW, Hartmann E (1978) Genetic and physiological studies of the effect of light on the development of the moss Physcomitrella patens. Photochem Photobiol 27: 249–254
Elzenga JTM, Prins HBA, Van Volkenburgh E (1995) Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta 197: 127–134
Graziana A, Fosset M, Ranjeva R, Hetherington AM, Lazdunski M (1988) Ca2+ channel inhibitors that bind to plant cell membrane block Ca2+ entry into protoplasts. Biochemistry 27: 764–768
Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ (1995) Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA 92: 4124–4128
Jabben M, Deitzer GF (1978) A method for measuring phytochrome in plants grown in white light. Photochem Photobiol 27: 799–802
Kamiya A (1994) Effects of far-red light on K+ fluxes in a colourless mutant of Chlorella. Plant Cell Physiol 35: 131–134
Knight CD, Cove DJ, Boyd PJ, Ashton NW (1988) The isolation of biochemical and developmental mutants in Physcomitrella patens. In: Glime JM (ed) Methods in bryology. Hattori Botanical Laboratory, Miyazaki, pp 47–58
Marshall J, Corzo A, Leigh RA, Sanders D (1994) Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J 5: 683–694
Miller AJ, Sanders D (1987) Depletion of cytosolic free calcium induced by photosynthesis. Nature 326: 397–400
Neuhaus G, Bowler C, Kern R, Chua N-H (1993) Calcium/ calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell 73: 937–952
Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D (1995) Phytochromes: Photosensory perception and signal transduction. Science 268: 675–680
Racusen RH, Satter RL (1975) Rhythmic and phytochrome-regulated changes in transmembrane potential in Samanea pulvini. Nature 255: 408–410
Roux SJ (1994) Signal transduction in phytochrome responses. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Academic Publishers, Amsterdam, pp 187–209
Rüdiger W, Thümmler F (1994) The phytochrome chromophore. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Academic Publishers, Amsterdam, pp 51–69
Russ U, Grolig F, Wagner G (1991) Changes of cytoplasmic free Ca2+ in the green alga Mougeotia scalaris as monitored with indo-1, and their effect on the velocity of chloroplast movements. Planta 184: 105–112
Schumaker KS, Gizinski MJ (1993) Cytokinin stimulates dihydropyridine-sensitive calcium uptake in moss protoplasts. Proc Natl Acad Sci USA 90: 10937–10941
Shacklock PS, Read ND, Trewavas AJ (1992) Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358: 753–755
Simon PE, Naef JB (1981) Light dependency of the cytokinin-induced bud initiation in protonemata of the moss Funaria hygrometrica. Physiol Plant 53: 13–18
Terry BR, Findlay GP, Tyerman SD (1992) Direct effects of Ca2+-channel blockers on plasma membrane cation channels of Amaranthus tricolor protoplasts. J Exp Bot 43: 1457–1473
Thaler M, Simonis W, Schönknecht G (1992) Light-dependent changes of the cytoplasmic H+ and Cl− activity in the green alga Eremosphaera viridis. Plant Physiol 99: 103–110
Thomine S, Zimmermann S, Van Duijn B, Barbier-Brygoo H, Guern J (1994) Calcium channel antagonists induce direct inhibition of the outward rectifying potassium channel in tobacco protoplasts. FEBS Lett 340: 45–50
Trebaçz K, Tarnecki R, Zawadzki T (1989) The effects of ionic channel inhibitors and factors modifying metabolism on the excitability of the liverwort Conocephalum conicum. Physiol Plant 75: 24–30
Trebacz K, Simonis W, Schönknecht G (1994) Cytoplasmic Ca2+, K+, Cl− and NO activities in the liverwort Conocephalum conicum L. at rest and during action potentials. Plant Physiol 106: 1073–1084
Tretyn A, Kendrick RE, Wagner G (1991) The role(s) of calcium ions in phytochrome action. Photochem Photobiol 54: 1135–1155
Tyerman SD (1992) Anion channels in plants. Annu Rev Plant Physiol Plant Mol Biol 43: 351–373
Wagner G, Klein K (1981) Mechanism of chloroplast movement in Mougeotia. Protoplasma 109: 169–185
Ward JM, Pei Z-M, Schroeder JI (1995) Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 7: 833–844
Wayne RO, Helper PK (1985) Red light stimulates an increase in intracellular calcium in the spores of Onoclea sensibilis. Plant Physiol 77: 8–11
Weisenseel MH, Ruppert HK (1977) Phytochrome and calcium ions are involved in light-induced membrane depolarisation in Nitella. Planta 137: 225–229
Author information
Authors and Affiliations
Corresponding author
Additional information
We thank Prof. David Cove (Department of Genetics, University of Leeds) for fruitful discussions, providing plants and advice on culturing methods, Dr. Richard Firn (York) for stimulating discussions, Ian Jennings (York) for technical advice on the electrophysiological apparatus, and Anna Bate (York) for looking after the plant cultures. Financial support was received from the Biotechnology and Biological Sciences Research Council (Grant P87/4043 to D.S. and Grant PDF/14 to E.J.) and The New Phytologist Trust (studentship support to E.E.).
Rights and permissions
About this article
Cite this article
Ermolayeva, E., Hohmeyer, H., Johannes, E. et al. Calcium-dependent membrane depolarisation activated by phytochrome in the moss Physcomitrella patens . Planta 199, 352–358 (1996). https://doi.org/10.1007/BF00195726
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195726