Abstract
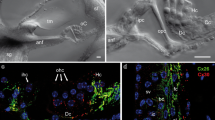
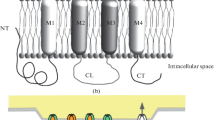
Gap junctions in the rat cochlea were investigated using immunostaining for connexin26 and transmission electron microscopy. Electron microscopy of normal and pre-embedded immunostained material showed that there were gap junctions between and among all cells that light microscopy showed to have immunostained appositions. Light microscopy showed immunostaining between and among all cell types that electron microscopy showed to be joined by gap junctions. Immunostaining for connexin26 was therefore taken as providing a reasonable approximation of the locations of gap junctions throughout the cochlea and was used to provide an overview of the extent of those locations. Cells interconnected via gap junctions fell into one of two groups. The first group consists of nonsensory epithelial cells and includes interdental cells of the spiral limbus, inner sulcus cells, organ of Corti supporting cells, outer sulcus cells, and cells within the root processes of the spiral ligament. The second group consists of connective tissue cells and includes various fibrocyte types of the spiral limbus and spiral ligament, basal and intermediate cells of the stria vascularis, and mesenchymal cells which line the scala vestibuli. The present work represents a first attempt towards a description of how serial gap junctions among cochlear cells reflect a level of organization of the tissue. The organization described here, together with a great deal of information from previous investigators, suggest that serially arranged gap junctions of both epithelial and connective tissue cells serve as the strucural basis for recycling endolymphatic potassium ions that pass through the sensory cells during the transduction process.
Similar content being viewed by others
References
Adams JC (1992) Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 40:1457–1463
Adams JC, Mugnaini E (1987) Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. J Comp Neurol 262:353–401
Bagger-Sjöbäck D, Engström B, Steinholtz L, Hillerdal M (1987) Freeze fracturing of the human stria vascularis. Acta Otolaryngol (Stockh) 103:64–72
Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Sáez JC (1991) Gap junctions: new tools, new answers, new questions. Neuron 6:305–320
Beyer EC, Paul DL, Goodenough DA (1987) Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105:2621–2629
Carlisle L, Steel K, Forge A (1990) Endocochlear potential generation is associated with intercellular communication in the stria vascularis: Structural analysis in the viable dominant spotting mouse mutant. Cell Tissue Res 262:329–337
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–677
Forge A (1984) Gap junctions in the stria vascularis and effects of ethacrynic acid. Hear Res 13:189–200
Franke K (1978) Freeze fracture aspects of the spiral limbus. Arch Otorhinolaryngol 221:157–162
Franke K (1979) Fine structure of the tissue lining the cochlear perilymphatic space against the bony labyrinthine capsule. Arch Otorhinolaryngol 222:161–167
Franke KD, Reale E, Iurato S, Luciano L, Wermbter G, Pannese E (1975) Verbindungskomplexe an Zellen der Reissner-Membran in gefriergebrochenen Präparaten. Acta Otolaryngol (Stockh) 80:38–42
Goliger JA, Paul DL (1994) Expression of gap junction proteins Cx26, Cx31.1, Cx37, and Cx43 in developing and mature rat epidermis. Dev Dyn 200:1–13
Gulley RL, Reese TS (1976) Intercellular junctions in the reticular lamina of the organ of Corti. J Neurocytol 5:479–507
Haefliger JA, Bruzzone R, Jenkins NA, Gilbert DJ, Copeland NG, Paul DL (1992) Four novel members of the connexin family of gap junction proteins. J Biol Chem 267:2057–2064
Hama K, Saito K (1977) Gap junctions between the supporting cells in some acoustico-vestibular receptors. J Neurocytol 6:1–12
Hennemann H, Suchyna T, Lichtenberg-Frate H, Jungbluth S, Dahl E, Schwarz J, Nicholson BJ, Willecke K (1992) Molecular cloning and functional expression of mouse connexin40, a second gap junction gene preferentially expressed in lung. J Cell Biol 117:1299–1310
Hoh JH, John SA, Revel J-P (1991) Molecular cloning and characterization of a new member of the gap junction gene family, connexin-31. J Biol Chem 266:6524–6531
Ichimiya I, Adams JC, Kimura RS (1994a) Immunolocalization of Na+,K+-ATPase, Ca++-ATPase, calcium-binding proteins, and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngol (Stockh) 114:167–176
Ichimiya I, Adams JC, Kimura RS (1994b) Changes in immunostaining of cochleas with experimentally induced endolymphatic hydrops. Ann Otol Rhinol Laryngol 103:457–468
Iurato S, Franke K, Luciano L, Wermbter G, Pannese E, Reale E (1976a) Fracture faces of the junctional complexes in the reticular membrane of the organ of Corti. Acta Otolaryngol (Stockh) 81:36–47
Iurato S, Franke K, Luciano L, Wermbter G, Pannese E, Reale E (1976b) Intercellular junctions in the organ of Corti as revealed by freeze fracturing. Acta Otolaryngol (Stockh) 82:57–69
Jahnke K (1975a) Feinstruktur gefriergeätzter Zellmembran-Haftstellen der Stria vascularis. Anat Embryol 147:189–201
Jahnke K (1975b) The fine structure of the freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol (Stockh) [Suppl] 336:1–40
Johnstone BM, Patuzzi R, Syka J, Sykova E (1989) Stimulus-related potassium changes in the organ of Corti of guinea pig. J Physiol (Lond) 408:77–92
Kamibayashi Y, Oyamada M, Oyamada Y, Mori M (1993) Expression of gap junction proteins connexin 26 and 43 is modulated during differentiation of keratinocytes in newborn mouse epidermis. J Invest Dermatol 101:773–778
Kikuchi K, Hilding DA (1966) The development of the Stria vascularis in the mouse. Acta Otolaryngol (Stockh) 62:277–291
Kikuchi T, Adams JC, Paul DL, Kimura RS (1993) Distribution of gap junction protein (connexin26) in the rat cochlea. Abstracts of the 16th ARO Midwinter Meeting: 89
Marcus DC (1986) Non-sensory electrophysiology of the cochlea: stria vascularis. In: Altschuler RA, Hoffman DW, Bobbin RP (eds) Neurobiology of hearing: the cochlea. Raven Press, New York, pp 123–137
Melichar I, Syka J (1987) DC potentials in different cells of the stria vascularis measured in vitro. Hear Res 25:23–33
Metz L, Aoki A, Forsmann WG (1978) Studies on the ultrastructure and permeability of the hemotrichorial placenta. Cell Tissue Res 192:391–407
Musil LS, Goodenough DA (1991) Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junction plaques. J Cell Biol 115:1357–1374
Nadol JB (1978) Intercellular junctions in the organ of Corti. Ann Otol 87:70–80
Nadol JB, Mulroy MJ, Goodenough DA, Weiss TF (1976) Tight and gap junctions in a vertebrate inner ear. Am J Anat 147:281–302
Oesterle EC, Dallos P (1989) Intercellular recordings from supporting cells in the guinea-pig cochlea: AC potentials. J Acoust Soc Am 86:1013–1032
Oesterle EC, Dallos P (1990) Intercellular recordings from supporting cells in the guinea pig cochlea: DC potentials. J Neurophysiol 64:617–636
Offner FF, Dallos P, Cheatham MA (1987) Positive endocochlear potential: mechanism of production by marginal cells of stria vascularis. Hear Res 29:117–124
Reale E, Luciano L, Franke K, Pannese E, Wermbter G, Iurato S (1975) Intercellular junctions in the vascular stria and spiral ligament. J Ultrastruct Res 53:284–297
Risek B, Klier FG, Gilula NB (1992) Multiple gap junction genes are utilized during rat skin and their development. Development 116:639–651
Sakagami M, Fukazawa K, Murata J, Matsunaga T (1993) Morphological aspects of transport of potassium ion in the marginal cell. Acta Otolaryngol (Stockh) [Suppl] 501:63–65
Salt AN, Konishi T (1986) The cochlear fluids: perilymph and endolymph. In: Altschuler RA, Hoffman DW, Bobbin RP (eds) Neurobiology of hearing: the cochlea. Raven Press, New York, pp 109–122
Santos-Sacchi J (1991) Isolated supporting cells from the organ of Corti: some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res 52:89–98
Schulte BA (1993) Immunohistochemical localization of intracellular Ca-ATPase in outer hair cells, neurons and fibrocytes in the adult and developing inner ear. Hear Res 65:262–273
Schulte BA, Adams JC (1989) Distribution of immunoreractive Na+,K+-ATPase in gerbil cochlea. J Histochem Cytochem 37:127–134
Smith CA, Davis H, Deatherage BH, Gessert CF (1958) DC potentials of the membranous labyrinth. Am J Physiol 193:203–206
Somjen GG (1979) Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol 41:159–177
Spicer SS, Schulte BA (1991) Differentiation of inner ear fibrocytes according to their ionic transport-related activity. Hear Res 56:53–64
Spray DC, Bennett MVL (1985) Physiology and pharmacology of gap junctions. Annu Rev Physiol 47:281–303
Sterkers O (1985) Origin and electrochemical composition of endolymph in the cochlea. In: Drescher DG (ed) Audiology biochemistry. Thomas, Springfield, pp 473–487
Swenson KI, Piwnica-Worms H, McNamee H, Paul DL (1990) Tyrosine phosphorylation of the gap junction protein cornnexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regulation 1:989–1002
Takahashi T, Kimura RS (1970) The ultrastructure of the spiral ligament in the Rhesus monkey. Acta Otolaryngol (Stockh) 69:46–60
Willecke K, Hennemann H, Dahl E, Jungbluth S, Heynkes R (1991) The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol 56:1–7
Zhang JT, Nicholson BJ (1989) Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol 109:3391–3401
Zwislocki JJ, Slepecky NB, Cefaratti LK, Smith RL (1992) Ionic coupling among cells in the organ of Corti. Hear Res 57:175–194
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kikuchi, T., Kimura, R.S., Paul, D.L. et al. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol 191, 101–118 (1995). https://doi.org/10.1007/BF00186783
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186783