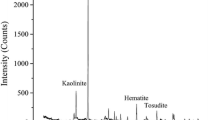
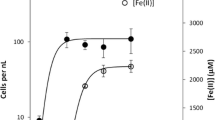
Abstract
The nutritional responses of unattached and attached bacterial communities were studied in groundwater from 3 sampling depths, i.e., 830–841 m, 910–921 m, and 999–1,078 m, of the subvertical borehole KLX01 at the Laxemar study area in SE Sweden. The salinity profile of the groundwater in this borehole is homogeneous. There were negative redox potentials (Eh) in the waters (−220 to −270 mV) and they contained sulfide, hydrogen, and methane. Biofilm reactors with hydrophilic glass surfaces were connected to the flowing groundwaters from each of the 3 depths with flow rates of approximately 3 x 10−3 m sec−1 over 19 days. There were 0.15 to 0.68 × 105 unattached bacteria ml−1 groundwater and 0.94 to 1.2 × 105 attached bacteria cm−2 on the surfaces. The assimilations of 14CO2, 14C-formate, 1,2,3-3H-acetate, U-14C-lactate, U-14C-glucose, and L-4,5-3H-leucine by the communities were demonstrated with microautoradiographic and liquid scintillation counting techniques. There were significant assimilations of CO2 by all communities, except for the unattached bacteria at the 910–921 m depth, indicating in situ production of organic carbon from carbonate. Assimilation of formate was detected in two communities, indicating the presence of bacteria able to substitute CO2 with formate. Acetate, lactate, and glucose assimilations demonstrated the presence of heterotrophic bacteria. The assimilation of lactate by the attached bacteria dominated over acetate and glucose at all depths. Leucine was assimilated by 20 to 98% of the communities, which showed that major portions of the communities studied were viable. The results indicate that the attached communities at the 830–841 m and 910–921 m depths were in more metabolically active states than the unattached bacteria. Incubation in air compared with N2 indicated that portions of the studied communities were obligate anaerobes, as their ability to assimilate the added compounds was sensitive to oxygen. The results show that the use of several different compounds reduces the risk for false conclusions about the viability and the metabolic activity of the deep groundwater communities.
Similar content being viewed by others
References
Barker JF, Fritz P (1982) The occurrence and origin of methane in some ground water flow systems. Can J Earth Sci 18:1802–1816.
Belyaev SS, Ivanov MV (1983) Bacterial methanogenesis in undergroundwaters. In: Hallberg R (ed) Environmental biogeochemistry. Proc 5th Int Symp Env Biogeochem (ISEB), Liber Tryck, Stockholm, pp 273–280.
Belyaev SS, Wolkin R, Kenealy WR, DeNiro MJ, Epstein SW, Zeikus JG (1983) Methanogenic bacteria from the Bondyuzhshoe oil field: General characterization and analysis of stable-carbon isotopic fractionation. Appl Environ Microbiol 45:691–697.
Beveridge TJ, Fyfe WS (1985) Metal fixation by bacterial cell walls. Can J Earth Sci 22:1893–1898.
Chapelle FH, Morris PB, McMahon PB, Zelibor JL (1988) Bacterial metabolism and the δ13C composition of groundwater, Floridan aquifer system, South Carolina. Geology 16:117–121.
Chapelle FH, Zelibor JLJ, Grimes DJ, Knobel LL (1987) Bacteria in deep coastal plain sediments of Maryland: A possible source of CO2 to groundwater. Wat Resour Res 23:1625–1632.
Fauque G, Legall J, Barton LL (1991) Sulfate-reducing and sulfur-reducing bacteria. In: Shively JM, Barton LL (eds) Variations in autotrophic life. Academic Press, London, pp 271–337.
Fuchs G (1986) CO2 fixation in acetogenic bacteria: Variations on a theme. FEMS Microbiol Rev 39:181–213.
Fuchs G (1990) Alternative pathways of autotrophic CO2 fixation. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria. Springer-Verlag, Berlin, Heidelberg, New York, pp 365–382.
Fuchs G, Länge S, Rude E, Schäfer SSR, Scholtz R, Stupperich E (1987) Autotrophic CO2 fixation in chemotrophic anaerobic bacteria. In: Verseveld HW, Duine JA (eds) Microbial growth on C1 compounds. Martin Nijhoff Publishers, Dordrecht, pp 39–43.
Ghiorse WC, Wilson JT (1988) Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol 33:107–172.
Godsy EM (1980) Isolation of Methanobacterium bryantti from a deep aquifer by using a novel broth-antibiotic disk method. Appl Environ Microbiol 39:1074–1075.
Gustavsson G, Liedholm M, Rhén I, Stanfors R, Wikberg P (1991) Äspö hard rock laboratory. Predictions prior to excavation and the process of their validation. SKB technical report, 91–23, Stockholm. Swedish Nuclear Fuel and Waste Management Co (Box 5864, S-102 48 Stockholm).
Gustafson G, Stanfors R, Wikberg P (1988) Swedish hard rock laboratory first evaluation of preinvestigations 1986–1987 and target area characterization. SKB technical report, 88–16, Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Gustafson G, Stanfors R, Wikberg P (1989) Swedish hard rock laboratory first evaluation of preinvestigations 1988 and target area characterization. SKB technical report, 89–16, Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Hall GH, Jones JG, Pickup RW, Simon BM (1990) Techniques for estimating bacterial growth rates and production of biomass in aquatic environments. In: Grigorova R, Norris JR (eds) Methods in microbiology, vol. 22. Academic Press, London, pp 181–209.
Hallbeck L, Pedersen K (1990) Culture parameters regulating stalk formation and growth rate of Gallionella ferruginea. J Gen Microbiol 136:1675–1680.
Hicks RJ, Fredrickson JK (1989) Aerobic metabolic potential of microbial communities indigenous to deep subsurface environments. Geomicrobiol J 7:67–78.
Hobbie JE (1990) Measuring heterotrophic activity in plankton. In: Grigorova R, Norris JR (eds) Methods in microbiology, vol. 22. Academic Press, London, pp 235–250.
Kirchman D, K'nees E, Hodson R (1985) Leucine assimilation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49: 599–607.
Kuenen JG, Bos P (1989) Habitat and ecological niches of chemolitho(auto)trophic bacteria. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria. Springer-Verlag, Berlin, Heidelberg, New York, pp 53–96.
Ladd TI, Costerton JW (1990) Methods for studying biofilm bacteria. In: Grigorova R, Norris JR (eds) Methods in microbiology, vol. 22. Academic Press, London, pp 285–307.
Lakksoharju M (1990) Colloidal particles in deep Swedish granitic groundwater. SKB progress report, 90–37, Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Niemelä S (1983) Statistical evaluation of results from quantitative microbiological examinations. Nordic committee on food analysis. Report no 1, 2nd ed, ISSN 0281-5303.
Olson GJ, Dockins WS, McFeters GA (1981) Sulfate-reducing and methanogenic bacteria from deep aquifers in Montana. Geomicrobiol J 2:327–340.
Ormeland RS (1988) Biogeochemistry of methanogenic bacteria. Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 641–705.
Pedersen K (1982) Method for studying microbial biofilms in flowing-water systems. Appl Environ Microbiol 43:6–13.
Pedersen K, Albinson Y (1991) Effect of cell number, pH and lanthanide concentration on the sorption of promethium by Shewanella putrefaciens. Radiochim Acta 54:91–95.
Pedersen K, Ekendahl S (1990) Distribution and activity of bacteria in deep granitic groundwaters of southeastern Sweden. Microb Ecol 20:37–52.
Pedersen K, Holmström C, Olsson A-K, Pedersen A (1986) Statistical evaluation of the influence of species variation, culture conditions, surface wettability and fluid shear on attachment and biofilm development of marine bacteria. Arch Microbiol 145:1–8.
Petterson C, Ephraim J, Allard B, Bóren H (1990) Characterization of humic substances from deep groundwaters in granitic bedrock in Sweden. SKB technical report, 90–29, Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Swedish Standard Commission. Available from: Standard commission in Sweden, Box 3295, 103 66 Stockholm, Sweden.
Stanfors R, Erlström M, Markström I (1991) Äspö hard rock laboratory. Overview of the investigations 1986–1990. SKB technical report, 91–20, Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Strandberg GW, Starling E, Schumate H, Parrott JR (1981) Microbial cells as biosorbents for heavy metals: Accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aerunginosa. Appl Environ Microbiol 41:237–245.
Tabor PS, Neihof RA (1982) Improved MARG method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol 44:945–953.
Torstensson BA (1984) A new system for groundwater monitoring. Groundwater Monit Rev 3:131–138.
West JM, Christofi N, McKinley IG (1985) An overview of recent microbiological research relevant to the geological disposal of nuclear waste. Radioactive Waste Management and Nuclear Fuel Cycle 6:79–95.
Widdel F (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 469–585.
Wikberg P, Axelsen K, Fredlund F (1987) Deep groundwater chemistry. SKB technical report, 87–07 Stockholm. Swedish Nuclear Fuel and Waste Management Co.
Wood HG, Ljungdahl LG (1991) Autotrophic character of the acetogenic bacteria. In: Shively JM, Barton LL (eds) Variations in autotrophic life. Academic Press, London, pp 201–250.
Author information
Authors and Affiliations
Additional information
Offprint requests to: K. Pedersen.
Rights and permissions
About this article
Cite this article
Pedersen, K., Ekendahl, S. Assimilation of CO2 and introduced organic compounds by bacterial communities in groundwater from southeastern Sweden deep crystalline bedrock. Microb Ecol 23, 1–14 (1992). https://doi.org/10.1007/BF00165903
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00165903