Abstract
This article describes an approach for training a variety of species to learn the abstract concept of same/different, which in turn forms the basis for testing proactive interference and list memory. The stimulus set for concept-learning training was progressively doubled from 8, 16, 32, 64, 128 . . . to 1,024 different pictures with novel-stimulus transfer following learning. All species fully learned the same/different abstract concept: capuchin and rhesus monkeys learned more readily than pigeons; nutcrackers and magpies were at least equivalent to monkeys and transferred somewhat better following initial training sets. A similar task using the 1,024-picture set plus delays was used to test proactive interference on occasional trials. Pigeons revealed greater interference with 10-s than with 1-s delays, whereas delay time had no effect on rhesus monkeys, suggesting that the monkeys’ interference was event based. This same single-item same/different task was expanded to a 4-item list memory task to test animal list memory. Humans were tested similarly with lists of kaleidoscope pictures. Delays between the list and test were manipulated, resulting in strong initial recency effects (i.e., strong 4th-item memory) at short delays and changing to a strong primacy effect (i.e., strong 1st-item memory) at long delays (pigeons 0-s to 10-s delays; monkeys 0-s to 30-s delays; humans 0-s to 100-s delays). Results and findings are discussed in terms of these species’ cognition and memory comparisons, evolutionary implications, and future directions for testing other species in these synergistically related tasks.
Similar content being viewed by others
Tests of animal cognition were for many years conducted in matching-to-sample tasks with commercial projector units, typically containing 12 stimuli (color shapes, line tilts, etc.). Samples (e.g., red circle) were typically presented in the middle of a stimulus panel facing the animal. Following a sample response, two choice stimuli (e.g., red circle, green circle) were presented on either side of the sample; a response to the choice stimulus that matched the sample was correct and reinforced. Animals (pigeons, monkeys, etc.) learned the matching task (or oddity task where they chose the nonmatching stimulus, e.g., green circle). But evidence of learning an abstract concept (i.e., transfer to novel stimuli) of matching or oddity was elusive. Some concept learning occurred when stimuli were junk objects placed on sliding trays in the Wisconsin General Test Apparatus (e.g., Mishkin & Delacour, 1975). One possibility was that the concept learning difference might be due to using real objects instead of synthetic picture stimuli like colored geometrical forms.
About this same time, David Premack made a profound claim that animals without language training (unlike humans and some apes) could not learn a same/different abstract concept (Premack 1978, 1983; Premack & Premack, 1983). The same/different task is similar to the matching-to-sample task in that single sample stimuli are presented, but the test contains only one stimulus, either a matching stimulus or a nonmatching stimulus. The response is either a same response or a different response (as defined in the task). The ability to learn the same/different abstract concept was promulgated to be the defining measure of cognitive ability and intelligence, and it was backed up by considerable evidence from studies examining human cognitive development in terms of the ability to understand equivalence, conservation of area, volume, and number (Daehler & Bukatko, 1985; Marcus, Vijayan, Rao, & Vishton, 1999; Piaget & Inhelder, 1966/1969; Siegler, 1996) and the ability to construct novel sentences and novel sequences of mathematical operations (e.g., Chen & Mo, 2004; Christie, Gentner, Call, & Haun, 2016; Smith, Langston, & Nisbett, 1992). Moreover, learning the same/different abstract concept was claimed to extend into adulthood forming the “very keel and backbone of our thinking,” as William James (1890/1950, p. 459), 128 years ago, proclaimed. Unfortunately, there were little or no breakthroughs for many years in training (most) nonhuman animals to learn abstract concepts.
Attempts to study nonhuman animal memory in similar tasks also came up against similar “road blocks” in obtaining adequate performance. Results from some memory studies using matching-to-sample tasks where delays were imposed between the sample stimulus and the choice stimuli are shown in Fig. 1. All studies in Fig. 1 used only two (different) stimuli to test either monkeys (capuchin, rhesus) or pigeons (Etkin & D’Amato, 1969; Moise, 1976; Overman & Doty, 1980; Roberts & Grant, 1976). At a zero-second delay (the choice stimuli appeared just as the sample was removed) accuracy was quite high, more than 80% correct. With longer delays accuracy fell to near chance (50% correct) performance in 30 s to 60 s.
At the time, implications from these results were that 30 to 60 s was the duration that information could be held in short-term (working) memory for capuchin monkeys, rhesus monkeys, and pigeons. But if this was true, then how could such animals survive in the natural environment? In the natural environment, these species would need to remember such things as new food sources (e.g., during migration), new members joining a flock or a troop, or new locations of predators over days and, months, let alone 1 minute.
The breakthrough that resolved whether 30 s to 60 s was really the limit of short-term memory for capuchin monkeys, rhesus monkeys, and pigeons was also the breakthrough for explaining why many nonhuman animals could not learn abstract concepts. In 1980, Overman and Doty further expanded upon their two-item matching-to-sample task with rhesus monkeys that was shown in Fig. 1. When they expanded the number of stimuli from two stimuli to 100 stimuli, so that 50 unique trials could be tested each session, then the monkeys’ memory accuracy was better than 85% correct (see Fig. 2, hexagons) after a 30-s delay, compared to only 53% accuracy when just two stimuli were tested (see Fig. 2, circles). Moreover, an additional expansion to completely novel stimuli in each testing session further increased memory performance (see Fig. 2, diamonds). After a 3-min delay, memory in the novel-stimulus condition was still better than 80% correct, and after a 24-hour delay better than 70% correct, a remarkable finding by any standards.
This better memory performance that increased as a function of the number of stimuli raises the issue of what was causing memory to be so limited to a few seconds when smaller numbers of stimuli had been used. The answer is proactive interference (not memory decay of the stimulus). Proactive interference occurs in matching to sample when a previously seen sample picture is re-presented as a nonmatching test picture. Having seen that test picture before, possibly as a sample stimulus in the immediately previous trial, tends to create confusion and increases the likelihood that a (incorrect) response will be made to the nonmatching picture. Notwithstanding several animal-memory investigations of proactive interference in the 1970s, the focus of those studies had been primarily on interference from the immediately preceding trial (e.g., Roberts & Grant, 1976, Fig. 1; see Wright, 2007; Wright, Urcuioli, & Sands, 1986, for other references). Moreover, many animal memory researchers did not fully appreciate the implications coming from human memory research. In a landmark study in 1962, Keppel and Underwood clearly demonstrated that proactive interference builds rapidly in memory tasks as a direct function of how many times the stimuli were repeated. They used a verbal memory task with humans, which may have contributed to it not being properly recognized by animal memory researchers. Nevertheless, as the stimuli were systematically repeated, proactive interference built rapidly producing dramatic declines in memory accuracy. The important implication for animal researchers was that by minimizing proactive interference, performance accuracy might then be sufficiently enhanced so that animals could learn high-order cognitive tasks, like a same/different abstract concept.
The previously mentioned landmark study by Overman and Doty (1980) did show such a result. The novel-stimulus test shown in Fig. 2 is more than just a demonstration of accurate memory performance; it is also a demonstration of abstract-concept learning. Novel-stimulus tests are the gold standard for abstract-concept learning. Therefore, the results from the novel-stimulus test in Fig. 2 are also a demonstration of matching-to-sample abstract-concept learning, a very important finding in itself. This important finding was a message to animal cognition researchers who tested animals for abstract-concept learning: Use novel stimuli or at least a very large stimulus set so that trial-unique stimuli could be presented for several successive testing sessions. All of these effects of obtaining good abstract-concept learning and single-item memory from nonhuman animals tested in matching-to-sample tasks, apply equally well to testing nonhuman animals in same/different tasks.
Despite this excellent single-item memory in the Overman and Doty study, real-world events are seldom encountered in isolation (single items). Instead, they are typically embedded within a string of other events. Strings of events need to be represented by list memory, not single-item memory. Serial list memory studies were among the first studies of memory (Ebbinghaus, 1902; Nipher, 1876). Many basic memory phenomena such as proactive and retroactive interference/inhibition, distinctiveness, long-term recency, repetition, and suffix effects require tests of list memory. Results from serial list memory studies are displayed as U-shaped serial position functions, showing accurate memory for the first list items (primacy effect), less accurate memory for middle items, and accurate memory for the last list items (recency effect). The serial list memory task is considered to be the “test bed” of memory theories (e.g., Glenberg, Bradley, Kraus, & Renzaglia, 1983), underscoring its prominent position in shaping thinking on how memory works.
Unlike the tradition of testing human list memory with recall procedures, animal list memory tests need to be conducted with recognition procedures. The same/different task has distinct advantages over the matching-to-sample task. In same/different tasks, the matching and nonmatching stimuli are not present at the same time as they are in matching-to-sample tasks. The advantage of separating trials on which matching and nonmatching stimuli occur is to eliminate relative comparisons between the two stimuli where choices can be made on the basis of the more familiar stimulus. In same/different tasks, lists of pictures can be presented to the animal followed by a test picture below the location where the list was presented. If the test item matches one of the list items, then a correct same response can be made to the test picture. If the test does not match any of the list items, then a correct different response can be made to a white rectangle located to the right of the test picture. Similar advantages apply to testing proactive interference in same/different tasks instead of matching-to-sample tasks. In same/different tasks, proactive interference occurs when a previously seen sample is re-presented as a test picture on a later different trial. Having previously seen that same test picture, possibly as a sample stimulus in the immediately previous trial, tends to create confusion and increases the likelihood that a same response (incorrect response) will be made.
Related to testing animal list memory in the same/different task is the serial-probe-recognition task or memory-scanning paradigm (Sternberg, 1966). Instead of a single memory item presented prior to the test as in delayed same/different tasks, a list of items is presented in serial-probe-recognition tasks prior to the test. If the test is identical to any one of the list items then the same response is correct, otherwise the different response is correct. Thus, the serial-probe-recognition task is an expanded version of the same/different task, and therefore an approach was necessary to train highly accurate performance in the same/different task.
Since the list-memory task is a direct extension of the simpler simultaneous same/different task, it seemed necessary to develop training techniques for full learning of the same/different abstract concept. A fully learned same/different abstract concept means that performance accuracy will be maintained when tested with virtually any number of novel stimuli. Moreover, such abstract-concept learning means that the set of testing stimuli could and should be very large (approaching infinitely large) without compromising accuracy of memory performance. A very large stimulus set minimizes and virtually eliminates proactive interference from repeating stimuli that occur when testing items on different trials had been seen previously in some recent list.
This important revelation of using a large stimulus set to minimize proactive interference forms the basis of successfully training animals to learn a same/different abstract concept, being able to successfully and directly test proactive interference, and ultimately to successfully test animals in the list-memory task. Large stimulus-set training was accomplished by gradually expanding the size of the training set, which resulted in increases in novel-stimulus transfer that eventually reached levels equivalent to training accuracy. Species comparisons in same/different abstract-concept learning will be shown for two nonhuman primate species and three avian species in the first section of this article. Following training in a delayed version of the same/different task, direct tests of proactive interference were made by systematically manipulating proactive interference to reveal its detrimental effects on same/different accuracy by pigeons and rhesus monkeys in the second section of this article. Following training with an expanding sample-list version of the same/different task and delay manipulations, serial-position functions and changes with delay will be shown for rhesus monkeys, capuchin monkeys, pigeons, and humans in the third section of this article. Relevance and importance of findings along with future plans will be discussed in each section in terms of how the rapid concept learning by corvids might relate to different mechanisms of proactive interference (i.e., the pigeon’s time-based proactive interference vs. the monkey’s event-based proactive interference) and might also relate to the different time-courses of list-memory changes (i.e., pigeon’s comparatively rapid change from recency memory to primacy memory vs. progressively slower changes for monkeys and humans, respectively).
Same/different abstract-concept learning
Same/different abstract-concept learning by monkeys
Due to the necessity of minimizing proactive interference, our focus shifted to procedures for accomplishing large stimulus-set training and determining how large a training set size would be needed for same/different abstract-concept learning across a variety of species. To insure that learning would proceed at a reasonable rate, we began with a small eight-item training set that would be successively doubled (logarithmic scale) to at least a set size that produced full concept learning (transfer accuracy with novel stimuli would be equivalent to accuracy on training trials). An advantage of using expanding training sets would be systematic variation of this critical variable (cf. Kamil, 1988). Moreover, whole functions relating the training set size to the degree of transfer would provide more powerful comparisons across species than transfer from any arbitrary selected training set size. Such an approach required a very large stimulus set, much larger than used previously, and the stimuli needed to be as distinctly different as possible. Therefore, so-called travel-slide pictures (e.g., scenes, objects, animals, buildings, people) were used (see Wright & Katz, 2006, for the stimuli used for training in each set size and stimulus pairs used for testing transfer after learning at each set size).
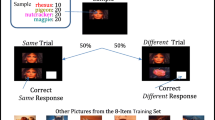
The first test with these procedures was conducted with three (experimentally naïve) capuchin (Cebus apella) monkeys (Wright, Rivera, Katz, & Bachevalier, 2003). Trials began with the simultaneous presentation of two pictures from a small training set of eight pictures, one above the other, plus a white rectangle in the lower right-hand corner (see Fig. 3). If the two pictures were the same, then a touch (choice) to the lower picture was correct; if the pictures were different, then a touch to the white rectangle in the lower right-hand corner was correct. Correct responses were reinforced with a banana pellet and followed by 15-s intertrial intervals. Incorrect responses were not reinforced and early in training were followed by a 15-s time-out and a repeat of the incorrect trial (correction procedure). Accuracy was based only on first trial performance. Each daily session contained 100 trials (50 same trials and 50 different trials). The capuchin monkeys learned this task in about 35 sessions (mean of 3,533 trials) and to a criterion of 80% correct or better on three consecutive sessions.
Examples of same and different trials. Capuchins were presented with both pictures and the white rectangle simultaneously, as shown in the second row. Rhesus touched the sample picture 10 times to get the lower picture and white rectangle. Pigeons, nutcrackers, and magpies pecked the sample picture 20 times to get the lower picture and white rectangle. A response to the bottom picture was correct on same trials and a response to the white rectangle was correct on different trials. The other six pictures of the initial 8-item training set are shown at the bottom
Rhesus monkeys (Macaca mulatta), by contrast, were not, for the most part, able to learn this task with the procedures used to train capuchin monkeys: one rhesus required 200 sessions to learn the task with the eight-item set, and two others showed little or no learning after 250 sessions. Therefore, these rhesus monkeys plus an additional three (experimentally naïve) rhesus monkeys were trained to touch the sample 10 times before being presented with the lower (test) item and the white rectangle (see Fig. 3); only then could they make their choice response (same response to the lower item, or different response to the white rectangle). With the 10-response requirement to the sample stimulus, all rhesus monkeys rapidly learned the eight-item set same/different task at a rate (40 training sessions) similar to capuchin monkeys with no sample-response requirement (Katz, Wright, & Bachevalier, 2002).
Following eight-item set learning, both monkey species were tested for transfer to novel stimuli. Transfer sessions consisted of 90 training (baseline) trials and 10 transfer trials (five same trials and five different trials). Picture stimuli on transfer trials were novel, and six transfer test sessions were conducted in consecutive daily sessions. Correct responses on transfer trials were reinforced similarly to correct responses on baseline trials. Incorrect responses were followed by the intertrial interval and the next trial. Transfer trials were intermixed with training trials, and trial sequences varied daily. Following transfer testing, the eight-item training set was doubled. Doubling the training set was repeated four times. Transfer testing was conducted following training with 32-item, 64-item, and 128-item set sizes when performance accuracy was 85% correct (or greater) on a single training session (see Fig. 4). Despite the response-requirement difference between monkey species, once the concept was learned then rhesus could perform as accurately as capuchins performed the same/different task without making sample-stimulus responses.
Mean percentage correct performance of three rhesus monkeys (red) and three capuchin monkeys (orange) on training (baseline) trials (broken lines, filled symbols) and novel (transfer) trials (unbroken lines, unfilled symbols) with successively expanded training sets from eight to 128 picture stimuli. The dotted line at 50% correct is chance performance. Error bars are + one standard error of the mean
Neither monkey species showed novel-picture transfer significantly different from chance (50% correct) performance following training with the initial eight-item training set in Fig. 4. But as the set size was increased to 32, 64, and 128 stimuli, transfer performance increased monotonically. Importantly, transfer performance accuracy following training with the 128-stimulus set was equivalent to baseline performance accuracy, revealing that both monkey species had fully learned the same/different abstract concept.
Control tests with other rhesus monkeys included a group where the set size was not expanded but the numbers of training sessions and transfer-testing sessions were otherwise matched to the experimental group. Transfer by this control group did not improve despite the same amount of training and transfer testing, thereby demonstrating that the manipulation of progressively expanding the training set and exponentially expanding the number of exemplars was the key to learning the same/different abstract concept.
Same/different abstract-concept learning by pigeons
Four pigeons (Columba livia) were trained and tested with procedures similar to those previously described for monkeys (Katz & Wright, 2006). Following successful training of rhesus monkeys that required 10 touches to the sample stimulus, it was deemed necessary to have pigeons perform a response requirement. Since pigeons peck more rapidly than monkeys touch, 20 pecks were required of pigeons before being presented with the test stimulus and white rectangle. Otherwise, trials and sessions were very similar to those used to train monkeys: 100 trial sessions (50 same, 50 different), the same training accuracy criteria, same transfer procedures with each of 6 transfer sessions containing 90 baseline (training) trials and 10 novel (five same, five different) transfer trials, the same training and testing stimuli, the same set-size expansions with training for three or more sessions with at least 85% correct. Training and testing were repeated six times for 32, 64, 128, 256, 512, and 1,024-item training sets, with the last three training sets being larger to maximize chances of obtaining full abstract-concept learning (see Fig. 5).
Mean percentage correct performance of four pigeons on training (baseline) trials (broken green lines, filled symbols) and novel (transfer) trials (unbroken green lines, unfilled symbols) with successively expanded training sets from eight to 1,024 picture stimuli in the same/different task. Dotted line at 50% correct is chance performance. Error bars are + one standard error of the mean
Mean percentage correct performance of seven Clark’s nutcrackers (blue) and 10 black-billed magpies (purple) on training (baseline) trials (broken lines and filled symbols) and novel (transfer) trials (solid lines, unfilled symbols) with successively expanded training sets from eight to 1,024 picture stimuli in the same/different task. Dotted line at 50% correct is chance performance. Error bars are + one standard error of the mean
Pigeons learned the initial eight-item task in about 30 sessions, similar to rhesus (40) and capuchin monkeys (35). Novel-stimulus transfer was similar (51.3%) to that of the monkeys and not different from chance (50%) correct performance. The pigeons’ transfer performance increased as the training set size increased and was equivalent to the training baseline levels for set sizes 256, 512, and 1,024, producing compelling evidence that pigeons can indeed fully learn the same/different abstract concept. Pigeons in a control group were trained without expansion of the eight-item training set size, but were yoked to individual experimental pigeons in terms of the number of training and transfer sessions. There was no increase in transfer performance for the control group, demonstrating that training set-size expansion was the critical manipulation to produce (full) concept learning. Thus, pigeons were shown to be able to fully learn the same/different abstract concept.
Although the pigeons’ full same/different abstract-concept learning is quite remarkable in itself, the set size required by pigeons (256-item set) was twice the size of that required by monkeys (128-item set). Indeed, the monkeys’ set-size transfer functions were nearly entirely above that for pigeons, showing that the rate of transfer growth occurred more rapidly and that the monkeys required many fewer exemplars of the same/different rule than did pigeons (numbers of rule exemplars grow as the square of set size). Such conclusions highlight the importance of comparing entire training set-size functions, particularly for an important cognitive function like abstract-concept learning that can be compared across different animal species (Wright, 2013). Nevertheless, pigeons, like monkeys, can and did attain full concept learning, which is a testament to using large training sets and the procedure of selecting training stimuli from the set without replacement to minimize stimulus repetitions across neighboring trials. If there had been stimulus repetitions from recent trials, then considerable proactive interference would have been produced, diminishing accuracy, hindering learning, and likely preventing full same/different concept learning. Before describing direct tests of how proactive interference builds with stimulus repetition, concept learning from two corvid species will be presented and discussed.
Same/different abstract-concept learning by nutcrackers and magpies
The conclusion that pigeons can fully learn the same/different abstract concept highlights the importance of entire functions for the different training set sizes to make meaningful comparisons across species. The additional power provided by comparing entire set-size functions for abstract-concept learning became readily apparent when trying to evaluate abstract-concept learning of a species, like Clark’s nutcrackers, that has a highly developed cognitive function of storing and successfully retrieving thousands of pine seeds months later when covered with snow in the winter or in the spring (Tomback, 1998; Vander Wall, 1982; Vander Wall & Balda, 1977). Such highly developed location memory depends upon precise relational memory for encoding the location of hundreds of cache sites relative to local landmarks, and executive decision-making. Encoding information in a relative manner can be shared across a variety of different tasks, including spatial and nonspatial tasks, and might show up as a qualitative cognitive difference between storing and nonstoring birds. Magpies are members of the corvid family and are closely related to nutcrackers, but they do not rely as strongly on cached food or make as many caches as do nutcrackers (Trost, 1999). Magpies inhabit more temperate altitudes and are more omnivorous than nutcrackers, thus providing a test for whether locating food caches by nutcrackers enhances same/different abstract-concept learning (retrieving cached seeds is also a relational-memory task), as well as a comparison to the nonstoring pigeon (not a member of the corvid family). This is where comparing entire concept-learning functions comes into play, facilitating direct comparisons among avian and primate species.
Seven wild-caught Clark’s nutcrackers (Nucifraga columbiana) and 10 wild-caught black-billed magpies (Pica hudsonia) were tested for their same/different abstract-concept learning using procedures very similar to those used to test pigeons including the same: stimuli, stimulus pairs, sequences of stimulus pairs used in training and transfer testing, display size, 15-s intertrial intervals, and required 20 pecks to sample stimuli (Magnotti, Katz, Wright, & Kelly, 2015; Magnotti, Wright, Leonard, Katz, & Kelly, 2017; Wright, Magnotti, Katz, Leonard, & Kelly, 2016; Wright, Magnotti, Katz, Leonard, Vernouillet, & Kelly, 2017). Nutcrackers and magpies made their pecks from a perch in front of the stimulus display. Similar to pigeons and monkeys, a response to the comparison picture was correct when it matched the sample picture; a response to the white rectangle to the right of the comparison picture was correct when the comparison picture did not match the sample picture. Correct choice responses were reinforced with mealworms delivered below the monitor via a rotating wheel. Like pigeons and monkeys, these birds were trained on 100-trial sessions (50 same, 50 different trials) and abstract-concept learning was assessed in six consecutive transfer sessions, each session contained 90 baseline (training) trials and 10 novel (five same, five different) transfer trials. Correct responses on transfer trials were reinforced identically to baseline trials. The cycle of set-size expansion, training for a minimum of three sessions, obtaining 85% correct or better, and novel-stimulus transfer testing was, like with pigeons, repeated six times for training sets of 32, 64, 128, 256, 512, and 1,024 picture items. (See Wright & Katz, 2006, for training and testing stimuli.)
Abstract-concept learning, as measured by transfer to novel picture pairs was 65% and 67% correct (chance 50% correct) following initial learning with the initial eight-item training set for nutcrackers and magpies, respectively (see Fig. 6). Transfer by both corvid species (Corvidae bird family) increased regularly and monotonically with the training set-size expansions until transfer performance was indistinguishable from baseline. Magpies and nutcrackers were statistically equivalent in their substantial transfer following training with the initial eight-item set, compared to the other species. Both of these corvid species clearly outperformed pigeons across the rising portion of the set-size functions. Nutcrackers and magpies also outperformed the monkeys in their initial transfer (partial concept learning) following training with the initial eight-item set. Like monkeys, nutcrackers and magpies attained full same/different abstract-concept learning with the 128-item training set size. Comparing learning rates on the initial acquisition, nutcrackers (3,300 trials) and magpies (3,500 trials) were very similar to the other species (rhesus, 4,000; capuchins, 3,500; and pigeons, 3,000 trials). Moreover, learning rates declined as the training set size was expanded from 16 to 1,024 items (magpies: 570, 480, 340, 320, 330, 340, 300 trials) and were similar for the other species demonstrating the benefit of progressively better transfer and partial concept learning (see also Wright & Katz, 2007).
Implications
Training and testing these different species with the same stimuli and procedures allowed direct species comparisons of how novel-stimulus transfer develops and systematically changes with training set size including: initial transfer with a small eight-item training set, the training set size where transfer is equivalent to baseline training performance, and the overall differences and similarities among the set-size transfer functions for different species. These comparisons were based on set-size manipulations that spanned the greatest portion of the set-size range producing entire functional relationships (Wright, 2013). Consider the implications had we only tested abstract-concept learning with eight-item training set. We might have incorrectly concluded that nutcrackers and magpies were able to learn (partially) the same/different abstract concept, but the other species were not. If we had stopped testing rhesus monkeys before initiating an observing response requirement, we might have incorrectly concluded that rhesus could not even learn the same/different discrimination. Functional relationships from systematic variation can also reveal fundamentally different cognitive mechanisms, as will be shown in the next section for proactive interference.
The nutcrackers’ and magpies’ set-size functions for same/different abstract-concept learning were virtually equivalent, including initial transfer (partial concept learning) and full concept learning, and therefore do not point to caching and recovery skills of nutcrackers being an advantage in same/different abstract-concept learning. The same can be said for the highly developed social skills of magpies. It appears that same/different abstract-concept learning is likely “baked” into the evolved neural architecture of nutcrackers and magpies. Moreover, the results from these two corvid species point to the possibility that corvids generally might be able to fully learn a higher-order abstract concept following exposure to a similar number of concept exemplars (128-picture set training) as either old-world (rhesus monkeys) or new-world (capuchin monkeys) nonhuman primates.
Modern lineages of birds and mammals evolved from survivors of a catastrophic asteroid event (Cretaceous–Paleogene extinction event) that wiped out all of the world’s big land animals (e.g., big land-living dinosaurs) some 66 million years ago (e.g., Alvarez, Alvarez, Asaro, & Michel, 1980; Schulte, 2010). Some small burrowing land animals survived, such as small feathered dinosaurs that evolved into modern birds, and small furry animals (e.g., monotremes, marsupials, placentals) that evolved into primates and other mammals. Body architectures, including brains, were and are very different for birds and mammals. Mammals, particularly primates, evolved large brains (compared to body weight) with folded neocortex, including the prefrontal cortex, and elaborate temporal lobe structures (e.g., hippocampus plus adjoining parahippocampal cortex), key structures for primate relational processing, abstract-concept learning, and episodic memory.
The “bird brain,” by contrast (like the demeaning use of the term), has until recently been thought to be primitive. Because most birds fly, light weight is required (e.g., hollow and trussed bones, lack of bladder). Nevertheless, many bird brains, and corvid brains in particular, are substantial in size and weight compared to their body weight with a well-developed hippocampus (e.g., Gould et al., 2013). But birds do not have a six-layer neocortex as do primates (birds have nodal/nuclear structures that may have some advantages in shorter connectivity and speed e.g., Clayton & Emery, 2015; Letzner, Güntürkün & Beste, 2017). Many functions of the mammalian prefrontal cortex have been found in the birds’ brain structure called the caudolateral nidopallium (e.g., Emery, 2006; Güntürkün, 2005; Kirsch, Güntürkün, & Rose, 2008; Viet & Nieder, 2013), a brain structure with tightly packed, high-density neurons (Olkowicz et al., 2016).
These very different brain architectures raise the issue of how the apparently primitive “bird brain” that evolved from dinosaurs became competitive with, and even initially outperformed, the accepted more elaborate primate brain in performing thoughts and processes considered of the highest cognitive order, same/different abstract-concept learning. The answer most certainly lies in evolution itself, a multimillion year process. Environmental pressures (social and otherwise) undoubtedly selected for and shaped these different neural architectures to successfully accomplish many of the same essential and intelligent behaviors for survival, an example of convergent evolution, where organisms not closely related (not monophyletic) independently evolved similar traits or functions as a result of having to adapt to similar environments or ecological niches. But the example of convergent evolution presented here is relatively unique due to being based on cognitive tests of fully learning a same/different abstract concept, whereas most other examples of convergent evolution are based on fossil records revealing obvious physical traits, like wings for an obvious function, like flying (by some insects, birds, and bats).
Whether same/different abstract-concept leaning is the only cognitive trait (domain specific) or part of a larger toolkit of cognitive traits (domain generality) necessary for such convergent evolution is not known. Nevertheless, birds and most other species frequently experience two-item same/different discriminations, for example, a female bird that is making a selection between two male birds, and chooses the male with the “prettier/sexier” feathers. Although other discriminations could involve more than two choices, such discriminations can and are broken down into a series of two-choice discriminations. The bottom line is that two-item same/different discriminations can underlie an array of cognitive discriminations and solutions (e.g., mate selection, food selection, predator avoidance). Thus, by itself two-item same/different discriminations could underlie and be a prime example of convergent evolution. Abstract same/different discriminations are so powerful and adaptive because the discrimination is not limited to memorizing a few differences (exemplars) and may have considerable generality across domains (as per the prescient proclamation by William James,1890/1950, p. 459).
Future directions
The two corvid species (nutcrackers and magpies) contributed considerably to what the so-called lowly bird brain can accomplish by achieving full same/different abstract-concept learning, a cognitive process of the highest order, somewhat more rapidly than two primate species. Similar tests of other avian species that are widely considered intelligent would likely expand upon this evidence of convergent evolution of abstract cognitive processing (see also Letzner et al., 2017).
Proactive interference
In order to make direct tests of proactive interference on the memory of previous individual trials, the same/different task is better suited than the delayed matching-to-sample task because matching and nonmatching test stimuli are presented on separate trials (same vs. different stimuli). In matching-to-sample tasks the subject is presented with two test stimuli on proactive interference (PI) test trials; one stimulus matches the current-trial sample stimulus, which can serve as a recognition reminder of the current sample stimulus. Whereas in same/different tasks the subject is presented with only one test stimulus on proactive interference test trials, which matches a sample stimulus of a previous trial and provides no reminder of the current sample stimulus. Having seen that test picture before, possibly as a sample in the immediately previous trial, creates confusion and increases the likelihood that a same response (incorrect response) will be made, as shown in the trial example of Fig. 7.
An example of trials used to test proactive interference. On the interference test trial (Trial n) the test stimulus does not match the sample stimulus on that trial, but it did match the sample stimulus on the previous trial (Trial n− 1) which tends to create confusion and increase the chances of making an incorrect same response to the test picture
Proper tests of proactive interference require inserting a few proactive interference test trials within a much larger session of no-interference trials (i.e., trial unique stimuli) so that random stimulus repetitions will not contaminate or disrupt the explicit tests of proactive interference. In these tests of proactive interference, the (potential) interfering stimulus could occur as a sample stimulus 1 to 16 trials prior to the test, thus providing a function of separation (time and trials) between the sample (interfering) stimulus and test stimulus. The greater the separation, the less interference will be produced.
Pigeons: Time-based proactive interference
Such a test of proactive interference was conducted with four pigeons (Wright, Katz, & Ma, 2012). A large stimulus set of 1,024 pictures (the same large picture set used to train same/different abstract-concept learning) was used in these proactive interference tests. Proactive interference was tested by placing potentially interfering stimuli either 1, 2, 4, 8 or 16 trials prior to test trials. Stimuli for baseline trials were trial-unique and not repeated for more than two weeks of testing. Pigeons pecked the sample stimulus 20 times, followed by a delay (1 s or 10 s, in a block design), a test stimulus and white rectangle, choice response, and a 15-s intertrial interval. Each daily session consisted of 64 trials with five interference tests (one test each at the one-trial, two-trial, four-trial, eight-trial, and 16-trial separations). There were 32 same and 32 different trials per session, 24 sessions per block, and four blocks alternating between 1-s and 10-s delays.
The shorter 1-s delay (red) produced considerable proactive interference particularly on the immediately preceding trial, and this proactive interference dissipated as the trial separation increased (see Fig. 8). With the longer 10-s delay (blue), there was a considerably larger 47% proactive interference effect when the interfering stimulus was presented in the immediately preceding trial. This greater proactive interference also dissipated with increasing trial separation, but there was still a residual interference effect of 11% for interfering stimuli presented 16 trials prior (and more than 6.5 min prior), which was considerably greater than that produced with the 1-s delay for interfering stimuli presented 16 trials prior.
Left: Signal detection theory model of elapsed time: Log time to the sample on the current (test) trial (log T C ) and log time to the interfering sample (log T I ). Right: Percentage correct performance for 1-s and 10-s delays, with model fits (1 sigma bands) based on the ratio (T C /T I ) of log times (Wright, Katz, & Ma, 2012)
Greater interference at the longer 10-s delay than at the shorter 1-s delay is, at least on first blush, counterintuitive. Interfering stimuli encountered more distantly in the past (144 s more distantly at n − 16 for 10 s vs. 1 s) should, according to models of decay or limited capacity, translate to more forgetting and therefore less interference. But just the opposite occurred. This counterintuitive finding was shown to obey a Weber–Fechner time ratio of time discriminations from the test to the current-trial sample stimulus divided by time to the interfering stimulus (see Wright et al., 2012). The model was fit simultaneously to both PI functions (colored shaded bands) using the same parameters (bias and maximum accuracy) accounting for 95% of the variance, including the no-PI condition.
Monkeys: Event-based proactive interference
Three rhesus monkeys were tested in same/different memory tasks for proactive interference (PI) from prior trials, similar to those used to test pigeons (Devkar & Wright, 2016). Most of the conditions were the same for monkeys as they were for pigeons including: trial-unique pictures selected from the same 1,024 picture set without replacement on baseline trials, interference tests separated by 1, 2, 4, 8 or 16 trials prior, 15-s intertrial intervals, numbers of trials per sessions, sessions per block, and repetitions of blocks. Monkeys were tested with three delays (1 s, 10 s, and 20 s); the longer 20-s delay was used to further push the time limits of time-based proactive interference shown by pigeons (see Fig. 9).
The three monkeys’ mean percentage-correct performance on trial blocks with 1-s, 10-s, or 20-s delays between the offset of sample presentation and the onset of the test presentation (n − 1 was the immediately preceding trial) compared with baseline performance (No-PI trials). Chance performance was 50% correct. Error bars are + one standard error of the mean (Devkar & Wright, 2016)
There were, however, no statistically significant differences or interactions among the three PI functions, and thus there was no evidence for time-based proactive interference for rhesus monkeys. Further tests of time-based proactive interference were conducted by manipulating the intertrial interval. In separate testing sessions, either a shorter 5-s intertrial interval or a longer 15-s intertrial interval was used, coupled with a 20-s delay for enhancing any time-based proactive interference (according to the pigeons’ time-based proactive interference). The shorter 5-s intertrial interval decreased the time to the interfering stimulus by 10 s per trial (or 160 s for 16 trials). Therefore, any time-based proactive interference should have increased proportionately. Nevertheless, even with the very short 5-s intertrial intervals that were coupled with long 20-s delays produced no statistical differences between those two proactive interference functions (see Fig. 10). Despite the lack of any effect of time, these proactive interference functions revealed substantial proactive interference covering a range of 40% to 90% accuracy. Such large proactive interference effects coupled with a lack of any effect of substantial time manipulations serve to converge on the conclusion that the primates’ proactive interference must be event-based, not time-based. Event-based proactive interference is due to the items themselves (i.e., the number of intervening events) without regard to how long in the past those events occurred.
Mean performance for three monkeys with five different separations between an interfering stimulus and a test stimulus compared to no interference (no PI) baseline trials with 5-s and 15-s intertrial intervals (ITIs) and 20-s delays (Devkar & Wright, 2016)
Implications
Monkeys revealed considerable proactive interference, producing functions with large robust proactive-interference effects (see Figs. 9 and 10). But there was no evidence of any time-based proactive interference, unlike for the pigeons. This insensitivity to time, points to the primates’ proactive interference being “event”-based, not “time”-based. Separations between the interfering stimulus and test stimulus are functionally the intervening events of the trials themselves, not the amount of time that they consume. Some readers will have noticed the similarity of the distinction between event-based proactive interference versus time-based proactive interference to other well-known memory processing distinctions including: episodic versus familiarity memory, remember versus know judgements, and explicit versus implicit memory.
Future directions
Unpublished pilot work also suggests that humans may share the monkey’s “event”-based proactive interference. If substantiated, such a finding might suggest shared proactive-interference processes across primates, generally.
Following from the previous section that the two corvid bird species performed somewhat better than monkeys in the early stages of learning a same/different abstract concept raises an intriguing issue about whether nutcrackers and/or magpies might share event-based proactive interference with nonhuman primates or time-based proactive interference with pigeons, the other bird species. If the outcome would be the former, with nutcrackers and/or magpies sharing event-based proactive interference with nonhuman primates, then it might shed some light on the role of the dentate gyrus of the primate hippocampal formation in episodic memory because birds do not have a dentate gyrus (e.g., Bingman & Muzio, 2017).
Monkey, pigeon, and human list memory
List memory has been examined since the study of behavior and cognition began (Ebbinghaus, 1902). List-memory results are displayed as a serial position function, typically revealing best memory for memory items at the beginning of the list (the primacy effect) and at the end of the list (the recency effect)—a U-shaped serial position function. These serial position effects and changes over time (retention delay) have had a profound impact on concepts and theories of how memory works (e.g., Crowder, 1976). List memory studies provide more information about how memory works than single-item memory studies because events in the real world are seldom encountered in isolation and instead are imbedded in a surrounding stream of events. Also, surrounding events can interfere with memory (proactively or retroactively) or inhibit memory (e.g., retrieval of memory) of any single item or event. And surrounding events can provide a context that can actually enhance memory and memory retrieval as per episodic memory. In order to make substantial progress on comparing (nonhuman) animal memory to human memory, nonhuman animals need to be trained and tested in list memory tasks.
The problem in testing nonhuman animals in list-memory tasks was for decades the inability of nonhuman animals to accurately remember even single items longer than 60 s, as shown in Fig. 1. But that has all changed, after it was shown that repeating a small number of to-be-remembered items resulted in the build-up of proactive interference discussed in the previous section. By systematically increasing the number of testing items, memory accuracy improved dramatically, resulting in a wide variety of animals being able to learn an abstract concept of same versus different, which in turn provides the extended accuracy necessary for testing these same animals’ list memory accompanied by systematic changes in the delay between the list and the test.
Among the advantages of studying list memory as opposed to single-item memory is the ability to study changes in memory for different list positions while the retention interval is increased, much like the dissipation of the human recency effect. Originally, dissipation of the recency effect was thought to be due to a lack of rehearsal of those items. Moreover, the primacy effect was thought to be due to active rehearsal of the first list items as presentation of the list progressed (Atkinson & Shiffrin, 1968). Primacy effects had been considered to be unique to humans because no nonhuman animal was thought capable of active rehearsal, and for a considerable amount of time, none had shown primacy effects.
Contrary to common wisdom of the time that nonhuman animals were incapable of rehearsal and therefore could not produce serial-position primacy effects, a rhesus monkey was trained with 211 unique picture items (scenes, objects, people, animals, etc.) and tested with lists of 10 and 20 pictures. Each item of the 10-item list was presented for 1 s, with an 0.8-s interstimulus interval (ISI) and a 1-s retention interval (Sands & Wright, 1980a, b). If the test item (in the lower screen) matched any one of the list items shown in the upper screen, then a same response (right-lever movement) was correct (see Fig. 11). If it matched no list item, then a different response (left lever movement) was correct. These changes from a simultaneous same/different task to 10-item and 20-item serial probe recognition (SPR) task caused very little disruption of this monkey’s performance. Performance was 86% correct with 10-item lists, and was even a respectable 81% correct with 20-item lists.
Left: Schematic of a 10-item list-memory testing procedure. A monkey hand and arm is shown starting a trial by pressing downward on a lever. List pictures are then sequentially presented on an upper screen. Following a delay, a single test picture is presented on a lower screen. The subject moves the lever to the right, a correct response (same), indicating that the test picture was in the list. (Left-lever movements would indicate that the test picture was not in the list.) Right: Serial position functions for a rhesus monkey tested with 10-item and 20-item lists with primacy and recency effects showing good memory for the first and last list items, respectively
To further explore what was responsible for primacy and recency effects, subjects were tested with short four-item memory lists to accommodate pigeons that had difficulty with lists longer than four items (e.g., Santiago & Wright, 1984; Wright, 1999, 2007; Wright, Santiago, & Sands, 1984). Lists of four “travel slide” pictures were used to test rhesus monkeys, capuchin monkeys and pigeons (see Fig. 12, bottom). Lists of four kaleidoscope pictures were used to test humans (see Fig. 12, top) to avoid ceiling effects and level the “playing” field for comparisons to the (nonhuman) animals (Wright, 1999; Wright, Santiago, Sands, Kendrick, & Cook, 1985). Four pictures were each presented for 1 s with 1-s interstimulus intervals between list items, and one of six retention intervals that varied from zero to 100 seconds, depending on the species. Typically, retention intervals were fixed for 32 trials per block with two blocks with different retention intervals (one short and one long) tested daily. Four randomized blocks of the six retention intervals were typically tested for the four species.
The serial position functions for four different species are shown in Fig. 13. All species showed changes in their serial position functions with retention interval. At the shortest delay, the serial position function was upward sloping, showing virtually pure recency performance. As retention delay increased, a primacy effect appeared, giving the function its characteristic U-shape. At the longer delays, the recency effect dropped out, and the serial position function was downward-sloping, eventually showing nearly pure primacy performance. These similar qualitative pattern of changes occurred in the serial position functions of all species, but there were time-course differences across the species. The dissipation of the recency effect took place in about 30 seconds for rhesus and capuchin monkeys, 10 seconds for pigeons, and 100 seconds for humans. The primacy effect began to appear in only 1 or 2 seconds after the end of the list presentation and was somewhat more rapid for rhesus monkeys and pigeons than it was for capuchin monkeys and humans.
Serial position functions showing primacy and recency effects that change as a function of retention delay for monkeys, pigeons, and humans, the fourth item is the last list item. One group of rhesus monkeys (circles, left error bars) was trained and tested with digitized pictures, computer monitors, and touch screens. Another group of rhesus monkeys (squares, right error bars) was trained and tested with Carousel projectors and a response lever that moved in three directions. Mean group error bars are shown below each serial position function. Different-trial performance is shown to the right of each serial position function. Animals were tested with travel-slide pictures, and humans were tested with kaleidoscope patterns, as per Fig. 12
There are two sets of serial position functions for rhesus monkeys in Fig. 13. One group of three rhesus monkeys (circles, left error bars) was trained and tested similarly to the other species with digitized pictures, computer monitors, and touch screens. The other group of two rhesus monkeys (squares, right error bars) was trained and tested like the monkey shown in Fig. 11. Those monkeys sat in a primate chair and pushed down on a three-position lever (“T” pattern) to start trials and were tested with 3,000 different “travel slides” back projected on screens using Carousel projectors. Other procedural aspects were similar: list pictures were presented for 1 s on an upper back-projection screen with 1-s intervals between items. Lists were followed by a delay (0, 1, 2, 10, 20, or 30 s), and then a test picture appeared on a lower back-projection screen. Those monkeys then moved the lever right or left to indicate that the two pictures were either the same (right movement) or different (left movement). Here, too, there were equal numbers of same and different trials in each delay block and correct responses of either type were reinforced with a squirt of Tang® orange drink (Wright et al., 1985). Results from these two groups of rhesus monkeys are very similar, despite different experimental environments, methods of picture presentation, and responses. These similar results by different groups of rhesus monkeys tested with different methods of picture presentations (35-mm slides vs. digital pictures presented on a computer video monitor) and different responses (lever vs. touch screen) systems, are a testament to reproducibility of the rhesus monkey’s serial position functions for list memory.
Together, these systematic serial position function changes constrain the possible explanations for these changes in visual memory as a function of retention delay. Consider the consistent result that memory for the first list item (primacy effect) improves with retention delay. The appearance of the primacy effect as the delay progressed was surprising and is counterintuitive to the hypothesis that memory decays with time.
Implications
Nonhuman animals show the important characteristics of primacy and recency list-memory effects, as do humans. In addition, the four species tested in the four-item visual list-memory task showed similar dynamic changes in their primacy and recency effects as the retention delay was increased—a qualitative similarity across species with widely differing evolutionary histories and neural architectures. Quantitative differences across species were shown in the different time courses of these serial-position-effect changes with retention delay. If only one retention delay had been tested, then the conclusions would likely have been different. A single delay would have sampled a different point along the continuum of serial-position-function changes for the different species, and dissipation of the recency effect or appearance of the primacy effect would have been a chance finding.
Memory, according to most theories, is supposed to decay with time—a so-called law of disuse—otherwise known as forgetting. Such forgetting is often portrayed as a passive decay process—like the recency effect. The waning of the recency effect, like all forgetting, is a hallmark of most memory theories, particularly dual-store models. The popular and venerable dual-store models (e.g., modal model) claim that the recency effect represents short-term memory, which decays with time (e.g., Atkinson & Shiffrin, 1968; Gillund & Shiffrin, 1984; Haarman & Usher, 2001). Indeed, the passive decay of the recency effect in human memory contributed to the rising popularity of the study of short-term memory and the so-called cognitive revolution. The time course of the recency effect was supposed to be a measure of the short-term memory buffer. But even this (theoretical) concept has not survived the test of time. Recency effects have been shown for greatly extended time scales, such as recall of United States presidents (Roediger & Crowder, 1976) and rugby scores by pub patrons (Baddeley & Hitch, 1977). Notwithstanding any lingering debate over whether dissipation of the recency effect is brought about by passive decay, the same cannot be said about the primacy effect. The increase in primacy memory with retention delay is difficult for most memory theories to handle. If memory were to decay with time, then how can primacy memory (primacy effect) increase with delay time?
These similarities and differences in memory were made apparent by using short memory lists and investigating list memory over a substantial range of the effective retention delay. If longer lists (e.g., >10 items) had been used, then the early serial-position-function changes (e.g., emergence of the primacy effect) would have been lost because other items would have been presented during the time that this serial position function change was occurring. Other tests of human memory with short lists and difficult-to-code memory items (like kaleidoscope patterns) have shown similar serial-position-function changes (e.g., snowflake patterns: Neath, 1993a, b; Neath & Knoedler, 1994; Knoedler, Hellwig, & Neath, 1999; antique car drawings: Korsnes, 1995; Korsnes & Gilinsky, 1993; and even tastes: Daniel & Katz, 2018).
Importantly for this discussion was that the serial position functions for all these species changed in a similar pattern with memory delay, but with a time scale difference. These similar patterns of serial-position-function changes and the underlying mechanisms responsible for these changes were the products of considerable different brain architectures of a bird species (pigeons), two monkey species (new and old world), and humans, which is remarkable and not really expected. Since the distinction among these four species is the time scale for which items in the list are being remembered, then how does this finding reflect on accepted cognitive processing differences among these species? The so-called bird brain, long assumed to be inferior, should more quickly recover primacy memory (for short lists of stimuli or events) than should monkeys, and monkeys more so than humans. Said otherwise, does a longer time scale of primacy-memory recovery fit our common conception of greater intelligence?
Future directions
One possible way to test the role of time in primacy-memory recovery (and recency dissipation) would be to test birds that appear particularly intelligent, like the Clark’s nutcrackers and black billed magpies who were shown to learn an abstract same/different concept even somewhat better initially than either monkey species, and then attained full concept learning as equally rapid (in terms of training set size) as the monkeys did. The issue regarding list memory is whether nutcrackers and/or magpies would look more like monkeys in terms of their time course for serial-position-functions changes and a return to primacy, than like pigeons, or the other way around.
References
Alvarez, L. W., Alvarez, W., Asaro, F., & Michel, H. V. (1980). “Extraterrestrial cause for the Cretaceous–Tertiary extinction”. Science, 208, 1095–1108. https://doi.org/10.1126/science.208.4448.1095
Atkinson, R. C., & Shiffrin, R. M. (1968). Human memory: A proposed system and its control processes. In K. W. Spence & J. T. Spence (Eds.), The psychology of learning and motivation (Vol. 2, pp. 89–105). New York: Academic Press.
Baddeley, A. D., & Hitch, G. J. (1977). Recency reexamined. In S. Dornic (Ed.), Attention and performance (Vol. 6, pp. 647–667). Hillsdale: Erlbaum.
Bingman, V. P., & Muzio, R. N. (2017). Reflections on the structural-functional evolution of the hippocampus: What is the big deal about a dentate gyrus? Brain Behavior & Evolution, 90, 53–61. https://doi.org/10.1159/000475592
Chen, Z., & Mo, L. (2004). Schema induction in problem solving: A multidimensional analysis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30, 583–600.
Christie, S., Gentner, D., Call, J., & Haun, D. B. (2016). Sensitivity to relational similarity and object similarity in apes and children. Current Biology, 26, 531–535. https://doi.org/10.1016/j.cub.2015.12.054
Clayton, N. S., & Emery, N. J. (2015). Avian models for human cognitive neuroscience: A proposal. Neuron, 86, 1330–1342. https://doi.org/10.1016/j.neuron.2015.04.024
Crowder, R. G. (1976). Principles of learning and memory. Hillsdale: Erlbaum.
Daehler, M. W., & Bukatko, D. (1985). Cognitive development. New York: Knopf.
Daniel, T. A., & Katz, J. S. (2018). Primacy and recency effects for taste. Journal of Experimental Psychology: Learning, Memory, and Cognition. https://doi.org/10.1037/xlm0000437
Devkar, D. T., & Wright, A. A. (2016). Event based proactive interference by rhesus monkeys. Psychonomic Bulletin and Review, 23, 1474–1482. https://doi.org/10.3758/s13423-016-1005-x
Ebbinghaus, H. E. (1902). Grundzuge der Psychologie [Basic psychology]. Leipzig: Von Veit.
Emery, N. J. (2006). Cognitive ornithology: The evolution of avian intelligence. Philosophical Transactions of the Royal Society B, 361, 23–43.
Etkin, M., & D’Amato, M. R. (1969). Delayed matching-to-sample and short-term memory in the capuchin monkey. Journal of Comparative and Psychological Psychology, 69(3), 544–549.
Glenberg, A. M., Bradley, M. M., Kraus, T.A., & Renzaglia, G. J. (1983). Studies of the long-term recency effect: Support for a contextually guided retrieval hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 9, 231–255.
Gillund, G., & Shiffrin, R. M. (1984). A retrieval model for both recognition and recall. Psychological Review, 91, 1–67.
Gould, K. L., Gilbertson, K. E., Hrvol, A. J., Nelson, J. C., Seyfer, A. L., Brantner, R. M., & Kamil, A. C. (2013). Differences in relative hippocampus volume and number of hippocampus neurons among five corvid species. Brain Behavior Evolution, 81, 56–70. https://doi.org/10.1159/000345560
Güntürkün, O. (2005). The avian ‘prefrontal cortex’ and cognition. Current Opinion in Neurobiology, 15, 686–693.
Haarman, H., & Usher, M. (2001). Maintenance of semantic information in capacity-limited item short-term memory. Psychonomic Bulletin & Review, 8, 568–578.
James, W. (1950). The principles of psychology. New York: Dover. (Original work published 1890)
Kamil, A. C. (1988). A synthetic approach to the study of animal intelligence. In D. W. Leger (Ed.), Comparative perspective in modern psychology: Nebraska Symposium on Motivation (Vol. 35, pp. 230–257), Lincoln: University of Nebraska Press.
Katz, J. S., & Wright, A. A. (2006). Same/different concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 32, 80–86.
Katz, J. S., Wright, A. A., & Bachevalier, J. (2002). Mechanisms of same/different abstract-concept learning by rhesus monkeys (Macaca mulatta). Journal of Experimental Psychology: Animal Behavior Processes, 28, 358–368.
Keppel, G., & Underwood, B. J. (1962). Proactive inhibition in short-term retention of single items. Journal of Verbal Learning & Verbal Behavior, 1, 153–161.
Kirsch, J. A., Güntürkün, O., & Rose, J. (2008). Insight without cortex: lessons from the avian brain. Consciousness and Cognition, 17, 475–483.
Knoedler, A. J., Hellwig, K. A., & Neath, I. (1999). The shift from recency to primacy with increasing delay. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, 474–487.
Korsnes, M. S. (1995). Retention intervals and serial list memory. Perceptual and Motor Skills, 80, 723–731.
Korsnes, M. S., & Gilinsky, S. A. (1993). Aging and serial list picture memory. Perceptual and Motor Skills, 76, 1011–1014.
Letzner, S., Güntürkün, O., & Beste, C. (2017). How birds outperform humans in multi-component behavior. Current Biology, 27, R996–R998.
Magnotti, J. F., Katz, J. S., Wright, A. A., & Kelly, D. M. (2015). Superior abstract-concept learning by Clark’s nutcrackers (Nucifraga columbiana). Biology Letters, 11. https://doi.org/10.1098/rsbl.2015.0148
Magnotti, J. F., Wright, A. A., Leonard, K., Katz, J. S., & Kelly, D. M. (2017). Abstract-concept learning in Black-billed magpies (Pica hudsonia). Psychonomic Bulletin & Review, 24, 431–435.
Marcus, G. F., Vijayan, S., Bandi Rao, S., & Vishton, P. S. (1999). Rule learning by seven-month-old infants. Science, 283, 77–80.
Mishkin, M., & Delacour, J. (1975). An analysis of short-term visual memory in the monkey. Journal of Experimental Psychology: Animal Behavior Processes, 4, 326–334.
Moise, S. L. (1976). Proactive effects of stimuli, delays, and response position during delayed matching from sample. Animal Learning & Behavior, 4, 37–40.
Neath, I. (1993a). Contextual and distinctive processes and the serial position function. Journal of Memory and Language, 32, 820–840.
Neath, I. (1993b). Distinctiveness and serial position effects in recognition. Memory & Cognition, 21, 689–698.
Neath, I., & Knoedler, A. J. (1994). Distinctiveness and serial position effects in recognition and sentence processing. Journal of Memory and Language, 33, 776–795.
Nipher, F. E. (1876). On the distribution of numbers written from memory. Transactions of the Academy of St. Louis, 3, 79–80.
Olkowicz, S., Kocourek, M., Lučan, R.K., Porteš, M., Fitch, W.T., Herculano-Houzel, S., & Němec, P. (2016). Birds have primate-like numbers of neurons in the forebrain, Proceeding of the National Academy of Sciences of the United States of America, 113, 7255–7260.
Overman, W. H., & Doty, R. W. (1980). Prolonged visual memory in macaques and man. Neuroscience, 5, 1825–1831.
Piaget, J., & Inhelder, B. (1969). The psychology of the child (H. Weaver, Trans.). New York: Basic Books. (Original work published 1966)
Premack, D. (1978). On the abstractness of human concepts: Why it would be difficult to talk to a pigeon. In S. H. Hulse, H. Fowler, & W. K. Honig (Eds.), Cognitive processes in animal behavior (pp. 423–451). Hillsdale: Erlbaum.
Premack, D. (1983). Animal cognition. Annual Review of Psychology, 34, 351–362.
Premack, D., & Premack, A. J. (1983). The mind of an ape. New York: W.W. Norton & Co.
Roberts, W. A., & Grant, D. S. (1976). Studies of short-term memory in the pigeon using the delayed matching-to-sample procedure. In D. L. Medin, W. A. Roberts, & R. T. Davis (Eds.), Processes of animal memory. Hillsdale: Erlbaum.
Roediger, H. L., III, & Crowder, R. G. (1976). A serial position effect in recall of United States presidents. Bulletin of the Psychonomic Society, 8, 275–278.
Sands, S. F., & Wright, A. A. (1980a). Primate memory: Retention of serial list items by a rhesus monkey. Science, 209, 938–940.
Sands, S. F., & Wright, A. A. (1980b). Serial probe recognition performance by a rhesus monkey and a human with 10- and 20-item lists. Journal of Experimental Psychology: Animal Behavior Processes, 6, 386–396.
Santiago, H. C., & Wright, A. A. (1984). Pigeon memory: Same/different concept learning, serial probe recognition acquisition and probe delay effects in the serial position function. Journal of Experimental Psychology: Animal Behavior Processes, 10, 498–512.
Schulte, P. (2010). The Chicxulub Asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science, 327, 1214–1218.
Siegler, R. S. (1996). Emerging minds: The process of change in children’s thinking. New York: Oxford University Press.
Smith, E. E., Langston, C., & Nisbett, R. E. (1992). The case for rules in reasoning. Cognitive Science, 16, 1–40.
Sternberg, S. (1966). High-speed scanning in human memory. Science, 153, 652–654.
Tomback, D. F. (1998). Clark’s nutcracker (Nucifraga columbiana). The Birds of North America Online. Ithaca, NY: Cornell Lab of Ornithology. Available at https://birdsna.org
Trost, C. H. (1999). In A. Poole (Ed.), Black-billed Magpie (Pica hudsonia). The Birds of North America Online. Ithaca: Cornell Lab of Ornithology. Available at https://birdsna.org
Vander Wall, S. B. (1982). An experimental analysis of cache recovery in Clark’s nutcracker. Animal Behaviour, 30, 84–94.
Vander Wall, S. B., & Balda, R. P. (1977). Coadaptations of the Clark’s nutcracker and the pinyon pine for efficient seed harvest and dispersal. Ecological Monographs, 47, 89–111.
Viet, L., & Nieder, A. (2013). Abstract rule neurons in the endbrain support intelligent behavior in corvid songbirds. Nature Communications, 4. https://doi.org/10.1038/ncomms3878
Wright, A. A. (1999). Visual list memory in capuchin monkeys (Cebus apella). Journal of Comparative Psychology, 113, 74–80.
Wright, A. A. (2007). An experimental analysis of memory processing. Journal of the Experimental Analysis of Behavior, 88, 405–433.
Wright, A. A. (2013). Functional relationships for investigating cognitive processes. Behavioural Processes, 93, 4–24.
Wright, A. A., & Katz, J. S. (2006). Mechanisms of same/different concept learning in primates and avians. Behavioural Processes, 72, 234–254.
Wright, A. A., & Katz, J. S. (2007). The generalization hypothesis of abstract-concept learning: Learning strategies and related issues in Macaca mulatta, Cebus apella, and Columba livia. Journal of Comparative Psychology, 121, 387–397.
Wright, A. A., Katz, J. S., & Ma, W. (2012). How to be proactive about interference: Lessons from animal memory. Psychological Science, 23, 453–458. https://doi.org/10.1177/0956797611430096
Wright, A. A., Magnotti, J. F., Katz, J. S., Leonard, K., & Kelly, D. M. (2016). Concept learning set-size functions for Clark’s nutcrackers. Journal of the Experimental Analysis of Behavior, 105, 76–84. https://doi.org/10.1002/jeab.174
Wright, A. A., Magnotti, J. F., Katz, J. S., Leonard, K., Vernouillet, A., & Kelly, D. M. (2017). Corvids outperform pigeons and primates in learning a basic concept. Psychological Science, 28, 437–444.
Wright, A. A., Rivera, J. J., Katz, J. S., & Bachevalier, J. (2003). Abstract-concept learning and list-memory processing by capuchin and rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes, 29, 184–198.
Wright, A. A., Santiago, H. C., & Sands, S. F. (1984). Monkey memory: Same/different concept learning, serial probe acquisition, and probe delay effects. Journal of Experimental Psychology: Animal Behavior Processes, 10, 513–529.
Wright, A. A., Santiago, H. C., Sands, S. F., Kendrick, D. F., & Cook, R. G. (1985). Memory processing of serial lists by pigeons, monkeys, and people. Science, 229, 287–289.
Wright, A. A., Urcuioli, P. J., & Sands, S. F. (1986). Proactive interference in animal memory research. In D. F. Kendrick, M. Rilling, & R. Denny (Eds.), Theories of animal memory (pp. 101–125). Englewood Cliffs: Erlbaum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, A.A., Kelly, D.M. & Katz, J.S. Comparing cognition by integrating concept learning, proactive interference, and list memory. Learn Behav 46, 107–123 (2018). https://doi.org/10.3758/s13420-018-0316-3
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0316-3