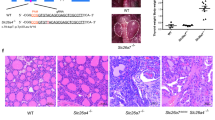
Abstract
Background: Mutations in the SLC26A4 gene, coding for the anion transporter pendrin, are responsible for Pendred syndrome, characterized by congenital sensorineural deafness and dyshormonogenic goiter. The physiological role of pendrin in the thyroid is still unclear and the lack of a thyroid phenotype in some patients with SLC26A4 mutations and in Slc26a4 (-/-) mice indicate the existence of environmental or individual modifiers able to compensate for pendrin inactivation in the thyroid. Since pendrin can transport iodide in vitro, variations in iodide supply have been claimed to account for the thyroid phenotype associated with pendrin defects. Aim: The Slc26a4 (-/-) mouse model was used to test the hypothesis that iodide supply may influence the penetrance and expressivity of SLC26A4 mutations. Materials and methods: Slc26a4 (-/-) and (+/+) mice were fed up to 6 months on a standard or low iodine diet and were evaluated for thyroid structural abnormalities or biochemical hypothyroidism. Results: A 27-fold iodide restriction induced similar modifications in thyroid histology, but no differences in thyroid size, T4 or TSH levels were observed between between Slc26a4 (-/-) and (+/+) mice, either in standard conditions and during iodine restriction. Conclusions: Iodide restriction is not able to induce a thyroid phenotype in Slc26a4 (-/-) mice. These experimental data, together with those coming from a review of familial Pendred cases leaving in regions either with low or sufficient iodide supply, support the idea that the expression of thyroid phenotype in Pendred syndrome is more powerfully influenced by individual factors than by dietary iodide.
Similar content being viewed by others
References
Pendred V. Deaf mutism and goitre. Lancet 1896, 148: 532.
Kopp P. Pendred’s syndrome: identification of the genetic defect a century after its recognition. Thyroid 1999, 9: 65–9.
Fugazzola L, Mannavola D, Cerutti N, et al. Molecular analysis of the Pendred’s syndrome gene and magnetic resonance imaging studies of the inner ear are essential for the diagnosis of true Pendred’s syndrome. J Clin Endocrinol Metab 2000, 85: 2469–75.
Bizhanova A, Kopp P. Genetics and phenomics of Pendred syndrome. Mol Cell Endocrinol 2010, Mar 15 [Epub ahead of print]; doi:10.1016/j.mce.2010.03.006.
Everett LA, Glaser B, Beck JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 1997, 17: 411–22.
Wangemann P, Nakaya K, Wu T, et al. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol 2007, 292: F1345–53.
Wangemann P, Itza EM, Albrecht B, et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2004, 2: 30.
Nakaya K, Harbidge DG, Wangemann P, et al. Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol 2007, 292: F1314–21.
Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol 2000, 278: C207–11.
Royaux IE, Suzuki K, Mori A, et al. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 2000, 141: 839–45.
Bidart JM, Mian C, Lazar V, et al. Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues. J Clin Endocrinol Metab 2000, 85: 2028–33.
Gillam MP, Sidhaye AR, Lee EJ, Rutishauser J, Stephan CW, Kopp P. Functional characterization of pendrin in a polarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem 2004, 279: 13004–10.
Rousset B. How many iodide transporters are there? how many true iodide transporter do we know? Hot Thyroidol 2006, June, e1.
Fugazzola L, Cerutti N, Mannavola D, et al. Differential diagnosis between Pendred and pseudo-Pendred syndromes: clinical, radiologic, and molecular studies. Pediatr Res 2002, 51: 479–84.
Everett LA, Belyantseva IA, Noben-Trauth K, et al. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 2001, 10: 153–61.
Delange FM. Iodine deficiency. In: LE Braverman and RD Utiger (eds). Werner & Ingbar’s The Thyroid. A fundamental and clinical text. 8th Edition. Lippincott Williams and Wilkins. 2000, 295–316.
Sandell EB, Kolthoff IM. Microdetermination of iodine by a catalytic method. Mikrochemica Acta 1937, 1: 9–25.
Benevenga NJ. Request for materials concerning the nutrient-requirements of laboratory-animals. J Nutrition 1991, 121: 1914.
Kim YH, Pham TD, Zheng W, et al. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol 2009, 297: F1069–79.
Pedraza PE, Obregon MJ, Escobar-Morreale HF, del Rey FE, de Escobar GM. Mechanisms of adaptation to iodine deficiency in rats: thyroid status is tissue specific. Its relevance for man. Endocrinology 2006, 147: 2098–108.
Royaux IE, Wall SM, Karniski LP, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 2001, 98: 4221–6.
Wall SM, Kim YH, Stanley L, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension 2004, 44: 982–7.
Verlander JW, Kim YH, Shin W, et al. Dietary Cl(-) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 2006, 291: F833–9.
Van Hauwe P, Everett LA, Coucke P, et al. Two frequent missense mutations in Pendred syndrome. Hum Mol Genet 1998, 7: 1099–104.
López-Bigas N, Rabionet R, de Cid R, et al. Splice-site mutation in the PDS gene may result in intrafamilial variability for deafness in Pendred syndrome. Hum Mutat 1999, 14: 520–6.
Kopp P, Arseven OK, Sabacan L, et al. Phenocopies for deafness and goiter development in a large inbred Brazilian kindred with Pendred’s syndrome associated with a novel mutation in the PDS gene. J Clin Endocrinol Metab 1999, 84: 336–41.
Bogazzi F, Raggi F, Ultimieri F, et al. A novel mutation in the pendrin gene associated with Pendred’s syndrome. Clin Endocrinol (Oxf) 2000, 52: 279–85.
Masmoudi S, Charfedine I, Hmani M, et al. Pendred syndrome: phenotypic variability in two families carrying the same PDS missense mutation. Am J Med Genet 2000, 90: 38–44.
Gonzalez Trevino O, Karamanoglu Arseven O, Ceballos CJ, et al. Clinical and molecular analysis of three Mexican families with Pendred’s syndrome. Eur J Endocrinol 2001, 144: 585–93.
López-Bigas N, Melchionda S, de Cid R, et al. Identification of five new mutations of PDS/SLC26A4 in Mediterranean families with hearing impairment. Hum Mutat 2001, 18: 548.
Blons H, Feldmann D, Duval V, et al. Screening of SLC26A4 (PDS) gene in Pendred’s syndrome: a large spectrum of mutations in France and phenotypic heterogeneity. Clin Genet 2004, 66: 333–40.
Napiontek U, Borck G, Müller-Forell W, et al. Intrafamilial variability of the deafness and goiter phenotype in Pendred syndrome caused by a T416P mutation in the SLC26A4 gene. J Clin Endocrinol Metab 2004, 89: 5347–51.
Pryor SP, Madeo AC, Reynolds JC, et al. SLC26A4/PDS genotypephenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet 2005, 42: 159–65.
Sugiura M, Sato E, Nakashima T, et al. Long-term follow-up in patients with Pendred syndrome: vestibular, auditory and other phenotypes. Eur Arch Otorhinolaryngol 2005, 262: 737–43.
Fugazzola L, Cirello V, Dossena S, et al. High phenotypic intrafamilial variability in patients with Pendred syndrome and a novel duplication in the SLC26A4 gene: clinical characterization and functional studies of the mutated SLC26A4 protein. EurJ Endocrinol 2007, 157: 331–8.
Pera A, Dossena S, Rodighiero S, et al. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci U S A 2008, 105: 18608–13.
Palos F, García-Rendueles ME, Araujo-Vilar D, et al. Pendred syndrome in two Galician families: insights into clinical phenotypes through cellular, genetic, and molecular studies. J Clin Endocrinol Metab 2008, 93: 267–77.
de Benoist B, Andersson M, Egli I, Takkouche B, Allen H. Iodine status worldwide. WHO Global Database on Iodine Deficiency. World Health Organization, Geneva 2004. http://whqlibdoc.who.int/publications/2004/9241592001.pdf
Reardon W, Coffey R, Chowdhury T, et al. Prevalence, age of onset, and natural history of thyroid disease in Pendred syndrome. J Med Genet 1999, 36: 595–8.
Scott DA, Wang R, Kreman TM, et al. Functional differences of the PDS gene product are associated with phenotypic variation in patients with Pendred syndrome and non-syndromic hearing loss (DFNB4). Hum Mol Genet 2000, 9: 1709–15.
Roepke TK, King EC, Reyna-Neyra A, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med 2009, 15: 1186–94.
Ibrahim IM, McDonald DO, Owen CJ, Kendall-Taylor P, Pearce SHS. Case report: Graves’ hyperthyroidism in a patient with Pendred’s dyshormonogenesis. Hot Thyroidol 2009, October: e13.
Yang T, Gurrola JG, 2nd, Wu H, et al. Mutations of KCNJ10 together with mutations of SLC26A4 cause digenic nonsyndromic hearing loss associated with enlarged vestibular aqueduct syndrome. Am j Hum genet 2009, 84: 651–7.
Yang T, Vidarsson H, Rodrigo-Blomqvist S, Rosengren SS, Enerback S, Smith RJ. Transcriptional Control of SLC26A4 Is Involved in Pendred Syndrome and Nonsyndromic Enlargement of Vestibular Aqueduct (DFNB4). Am J Hum Genet 2007, 80: 1055–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
DC and PP contributed equally to this work.
Rights and permissions
About this article
Cite this article
Calebiro, D., Porazzi, P., Bonomi, M. et al. Absence of primary hypothyroidism and goiter in Slc26a4 (-/-) mice fed on a low iodine diet. J Endocrinol Invest 34, 593–598 (2011). https://doi.org/10.3275/7262
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3275/7262