Abstract
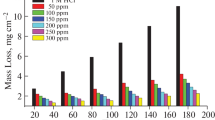
Potentiodynamic polarization, electrochemical impedance spectroscopy, mass loss, and hydrogen evolutions techniques were used to investigate the effect of the plant extract Cystoseira myrica as corrosion inhibitor for aluminum in 1 M HCl. All those measurements showed that the effectiveness of inhibition increased with an increase in the extract dose and in temperature. The inhibition efficiency of the extract reached 93.7% at 300 ppm, 25°C. Thermodynamic parameters of activation and adsorption were computed and discussed. Polarization curves showed that the extract studied behaves as mixed kind inhibitor. The Langmuir adsorption isotherm was obtained for the adsorption of the extract on the Al surface. Using the mass loss technique, it was revealed that adding KI enhances inhibitory efficiency from 77.3 to 91.6% owing to the synergistic effect. The surface analysis of aluminum was performed using different techniques. All other data approached were compatible and in line with each other.











Similar content being viewed by others
REFERENCES
Al-Abdali, F.H., Abdallah, M., and El-Sayed, R., Corrosion inhibition of aluminum using nonionic surfactant compounds with a six membered heterocyclic ring in 1.0 M HCl solution, Int. J. Electrochem. Sci., 2019, vol. 14, p. 3509.
Bashir, S., Sharma, V., Singh, G., Lgaz, H., et al., Electrochemical behavior, and computational analysis of phenylephrine for corrosion inhibition of aluminum in acidic medium, Metall. Mater. Trans. A, 2019, vol. 50, no. 1, p. 468.
Kurtela, M., Šimunović, V., Stojanović, I., and Alar, V., Effect of the cerium(III) chloride heptahydrate on the corrosion inhibition of aluminum alloy, Mater. Corros., 2020, vol. 71, p. 125.
Husaini, M., Usman, B., and Ibrahim, M.B., Study of corrosion inhibition of aluminum in nitric acid solution using anisaldehyde (4-methoxy benzaldehyde) as corrosion extract, Alg. J. Eng. Tech., 2019, vol. 1, p. 11.
Satyabama, P., Rajendran, S., and Nguyen, T.A., Corrosion inhibition of aluminum by oxalate self-assembling monolayer, Anti-Corros. Methods Mater., 2019, vol. 6, p. 768.
Imane, H., Mohamed, E., and Abdeslam, L., Inhibition of aluminum corrosion in 0.1 M Na2CO3 by Mentha palatium, Port. Electrochim. Acta, 2019, vol. 37, p. 335.
Fouda, A.S., El-Shereafy, E.E., Hathoot, A.A., and El-Bahrawi, N.M., Corrosion inhibition of aluminum by Cerumium rubrum extract in hydrochloric acid environment, J. Bio- Tribo-Corros., 2020, vol. 6, p. 1.
Abdallah, M., Al-Abdali, F.H., Kamar, E.M., El-Sayed, R., et al., Corrosion inhibition of aluminum in 1.0 M HCl solution by some nonionic surfactant compounds containing five membered heterocyclic moieties, Chem. Data Collect., 2020, vol. 28, ID 100407.
Song, W., Liang, G., Wei, C., Guo, W., et al., Synergistic effect of gamma-ray irradiation and calcium oxide on corrosion inhibition of aluminum dihydric tripolyphosphate on mild steel, Radiat. Eff. Defects Solids, 2020, vol. 175, p. 504.
Nazir, U., Akhter, Z., Ali, N.Z., and Shah, F.U., Experimental and theoretical insights into the corrosion inhibition activity of novel Schiff bases for aluminum alloy in acidic medium, RSC Advances, 2019, vol. 9, p. 36455.
Musa, H., Bishir, U., Adamu, I.M., and Bashir, I.M., Effect of aniline as corrosion extract on the corrosion of aluminum in hydrochloric acid solution, Res. J. Chem. Environ., 2020, vol. 24, p. 2.
Raghavendra, N., Ganiger, P.J., and Gaonkar, N.P., Application of expired Karthika Shampoo as corrosion extract for Al in 3 M HCl solution, Int. J. Eng. Tech. Manage. Res., 2019, vol. 3, no. 1, p. 1.
Prabhu, D., Prabhu, P.R., and Rao, P., Thermodynamics, adsorption, and response surface methodology investigation of the corrosion inhibition of aluminum by Terminalia chebula Ritz. extract in H3PO4, Chem. Pap., 2021, vol. 75, p. 653.
Fouda, A.S., Etaiw, S.H., El-Habab, A.T., and Wahba, A.M., Synthesis, characterization, and application of new nonionic surfactant as a corrosion extract for C-steel in 1 M hydrochloric acid solution, J. Bio- Tribo-Corros., 2020, vol. 6, ID 75. https://doi.org/10.1007/s40735-020-00373-8
Zhou, T., Yuan, J., Zhang, Z., Xin, X., et al., The comparison of imidazolium Gemini surfactant [C14-4-C14im] Br2 and its corresponding monomer as corrosion extracts for A3 carbon steel in hydrochloric acid solutions: Experimental and quantum chemical studies, Colloids Surf. A Physicochem. Eng. Asp., 2019, vol. 575, p. 57.
Sobhi, M., El-Sayed, R., and Abdallah, M., The effect of non ionic surfactants containing triazole, thiadiazole and oxadiazole as extracts of the corrosion of C-steel in 1 M hydrochloric acid, J. Surfactants Deterg., 2013, vol. 16, p. 937. https://doi.org/10.1007/s11743-013-1468-y
Fouda, A.S., Etaiw, S.H., and Wahba, A., Effect of acetazolamide drug as corrosion extract for carbon steel in hydrochloric acid solution, Nat. Sci., 2015, vol. 13, p. 1.
Deyab, M.A., Adsorption and inhibition effect of ascorbic palmitate on corrosion of carbon steel in ethanol blended gasoline containing water as a contaminant, Corros. Sci., 2014, vol. 80, p. 359.
Fuchs-Godec, R. and Pavlović, M.G., Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless steel X4Cr13 in sulphuric acid, Corros. Sci., 2012, vol. 58, p. 192.
Fouda, A.S., Migahed, H.E., Fouad, N., and Elbahrawi, N.M., Corrosion inhibition of carbon steel in 1 M hydrochloric acid solution by aqueous extract of Thevetia peruviana, J. Bio- Tribo-Corros., 2016, vol. 2, p. 16.
Farag, A.A., Ismail, A.S., and Migahed, M.A., Environmental-friendly shrimp waste protein corrosion extract for carbon steel in 1 M HCl solution, Egypt. J. Pet., 2019, vol. 27, p. 1187.
Al-Sabagh, A.M., Nasser, N.M., El-Azabawy, O.E., and El-Tabey, A.E., Corrosion inhibition behavior of new synthesized nonionic surfactants based on amino acid on carbon steel in acid media, J. Mol. Liq., 2016, vol. 219, p. 1078.
Sayed, A.R., Saleh, M.M., Al-Omair, M.A., and Abd Al-Lateef, H.M., Efficient route synthesis of new polythiazoles and their inhibition characteristics of mild steel corrosion in acidic chloride medium, J. Mol. Struct., 2019, vol. 1184, p. 452.
Sobhi, M., El-Sayed, R., and Abdallah, M., Synthesis, surface properties, and inhibiting action of novel nonionic surfactants on carbon steel corrosion in 1 M hydrochloric acid solution, Chem. Eng. Commun., 2016, vol. 203, p. 758.
Hermas, A.A. and Morad, M.S., A comparative study on the corrosion behaviour of 304 austenitic stainless steel in sulfamic and sulfuric acid solutions, Corros. Sci., 2008, vol. 50, no. 9, p. 2710. https://doi.org/10.1016/j.corsci.2008.06.029
Daoud, D., Douadi, T., Hamani, H., Chafaa, S., et al., Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study, Corros. Sci., 2015, vol. 94, p. 21.
Bedair, M.A., El-Sabbah, M.M.B., Fouda, A.S., and Elaryian, H.M., Synthesis, electrochemical and quantum chemical studies of some prepared surfactants based on azodye and Schiff base as corrosion extracts for steel in acid medium, Corros. Sci., 2017, vol. 128, p. 54.
Shalabi, K., Abdallah, Y.M., Hassan, H.M., and Fouda, A.S., Adsorption, and corrosion inhibition of Atropa belladonna extract on carbon steel in 1 M HCl solution, Int. J. Electrochem. Sci., 2014, vol. 9, p. 1468.
Aslam, R., Mobin, M., Zehra, S., Obot, I.B., et al., N, N'-Dialkylcystine gemini and monomeric N-alkyl cysteine surfactants as corrosion extracts on mild steel corrosion in 1M HCl solution: A comparative study, ACS Omega, 2017, vol. 2, p. 5691.
Basiony, N.E., Elgendy, A., Nady, H., Migahed, M.A., et al., Adsorption characteristics and inhibition effect of two Schiff base compounds on corrosion of mild steel in 0.5 M HCl solution: Experimental, DFT studies, and Monte Carlo simulation, RSC Advances, 2019, vol. 9, p. 10473.
Tasić, Ž.Z., Petrović Mihajlović, M.B., Radovanović, M.B., and Antonijević, M.M., New trends in corrosion protection of copper, Chem. Pap., 2019, vol. 73, no. 9, p. 2103. https://doi.org/10.1007/s11696-019-00774-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declare that they have no conflicts of interest.
About this article
Cite this article
Samar Y. Al-Nami Corrosion Inhibition of Aluminum in 1.0 M HCl Solution Using Cystoseira Myrica Extract. Surf. Engin. Appl.Electrochem. 58, 248–259 (2022). https://doi.org/10.3103/S1068375522030115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375522030115