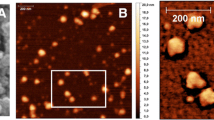
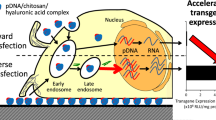
Abstract
One of the most popular nonviral delivery systems for gene therapy constructs are carriers based on polyethylenimine (PEI) DNA complexes. A number of disadvantages associated with the lack of targeted delivery and increased cytotoxicity are overcome by adding auxiliary molecules to the complexes. An example of this is chondroitin sulfate (CS). The purpose of this work was to assess the effect of CS on the transfection properties of DNA–PEI complexes under different conditions of their preparation and transfection protocols. All complexes were prepared in solutions with high and low ionic strength. Transfection of C26 cells was performed according to two protocols differing in the presence of serum in the medium. The portion of transfected cells, transgene expression level, and cell viability were the main parameters of assessing the transfection efficiency. In binary DNA–PEI complexes prepared in different salt conditions, using different transfection protocols, the difference in the portion of transfected cells reached ten times. Addition of CS improved this transfection efficiency indicator up to 6.5 times, while the maximum difference in this indicator for the corresponding ternary complexes was reduced to 2.5 times. Changes in the proportion of CS in the composition of the complexes had an insignificant effect on their transfection properties. In the case of complexes prepared in high ionic strength solutions, the order of CS addition was also important. The best results of transfection efficiency were achieved with ternary complexes prepared in low ionic strength solutions, using a serum-free protocol, while these indicators were comparable with the data for Lipofectamine 2000. The addition of chondroitin sulfate improves the transfection properties of DNA–PEI complexes and makes them less dependent on the methods of preparation and transfection.




Similar content being viewed by others
REFERENCES
Asad, A.S., Ayala, M.A.M., Gottardo, M.F., Zuccato, C., Javier, A., Candia, N., et al., Viral gene therapy for breast cancer: progress and challenges, Expert Opin. Biol. Ther., 2017, vol. 17, no. 8, pp. 945–959. https://doi.org/10.1080/14712598.2017.1338684
Samal, S.K., Dash, M., Van Vlierberghe, S., Kaplan, D.L., Chiellini, E., Van Blitterswijk, C., et al., Cationic polymers and their therapeutic potential, Chem. Soc. Rev., 2012, vol. 41, no. 21, pp. 7147–7194. https://doi.org/10.1039/c2cs35094g
Boussif, O., Lezoualc’h, F., Zanta, M., Mergny, M., Schermant, D., Demeneixt, B., et al., A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine, Proc. Natl. Acad. Sci. U. S. A., 1995, vol. 92, no. 16, pp. 7297–7301. https://doi.org/10.1073/pnas.92.16.7297
Kircheis, R., Wightman, L., and Wagner, E., Design and gene delivery activity of modified polyethylenimines, Adv. Drug Delivery Rev., 2001, vol. 53, no. 3, pp. 341–358. https://doi.org/10.1016/S0169-409X(01)00202-2
Hall, A., Lächelt, U., Bartek, J., Wagner, E., and Moghimi, S.M., Polyplex evolution: understanding biology, optimizing performance, Mol. Ther., 2017, vol. 25, no. 7, pp. 1–15. https://doi.org/10.1016/j.ymthe.2017.01.024
Ruponen, M., Ylä-Herttuala, S., and Urtti, A., Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: Physicochemical and transfection studies, Biochim. Biophys. Acta,Biomembr., 1999, vol. 1415, no. 2, pp. 331–341. https://doi.org/10.1016/S0005-2736(98)00199-0
Plank, C., Mechtler, K., Szoka, F.J., and Wagner, E., Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery, Hum. Gene Ther., 1996, vol. 7, no. 12, pp. 1437–1446. https://doi.org/10.1089/hum.1996.7.12-1437
Coll, J., Chollet, P., Brambilla, E., Desplanques, D., Behr, J., and Favrot, M., In vivo delivery to tumors of DNA complexed with linear polyethylenimine, Hum. Gene Ther., 1999, vol. 10, no. 10, pp. 1659–1666. https://doi.org/10.1089/10430349950017662
Ogris, M., Brunner, S., Schu, S., Kircheis, R., and Wagner, E., PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery, Gene Ther., 1999, vol. 6, no. 4, pp. 595–605. https://doi.org/10.1038/sj.gt.3300900
Ulasov, A.V., Khramtsov, Y.V., Trusov, G.A., Rosenkranz, A.A., Sverdlov, E.D., and Sobolev, A.S., Properties of PEI-based polyplex nanoparticles that correlate with their transfection efficacy, Mol. Ther., 2011, vol. 19, no. 1, pp. 103–112. https://doi.org/10.1038/mt.2010.233
Alekseenko, I.V., Snezhkov, E.V., Chernov, I.P., Pleshkan, V.V., Potapov, V.K., Sass, A.V., et al., Therapeutic properties of a vector carrying the HSV thymidine kinase and GM-CSF genes and delivered as a complex with a cationic copolymer, J. Transl. Med., 2015, vol. 13, no. 1, pp. 1–16. https://doi.org/10.1186/s12967-015-0433-0
Pathak, A., Kumar, P., Chuttani, K., Jain, S., Mishra, A.K., Vyas, S.P., et al., Gene expression, biodistribution, and pharmacoscintigraphic evaluation of chondroitin sulfate-PEI nanoconstructs mediated tumor gene therapy, ACS Nano, 2009, vol. 3, no. 6, pp. 1493–1505. https://doi.org/10.1021/nn900044f
Kurosaki, T., Kitahara, T., Kawakami, S., Nishida, K., Nakamura, J., Teshima, M., et al., The development of a gene vector electrostatically assembled with a polysaccharide capsule, Biomaterials, 2009, vol. 30, no. 26, pp. 4427–4434. https://doi.org/10.1016/j.biomaterials.2009.04.041
Ito, T., Iida-Tanaka, N., and Koyama, Y., Efficient in vivo gene transfection by stable DNA/PEI complexes coated by hyaluronic acid, J. Drug Targeting, 2008, vol. 16, no. 4, pp. 276–281. https://doi.org/10.1080/10611860801900728
Underhill, C., CD44: The hyaluronan receptor, J. Cell Sci., 1992, vol. 103, pp. 293–298.
Chen, C., Zhao, S., Karnad, A., and Freeman, J.W., The biology and role of CD44 in cancer progression: therapeutic implications, J. Hematol. Oncol., 2018, vol. 11, no. 1, pp. 1–23. https://doi.org/10.1186/s13045-018-0605-5
Kinugasa, Y., Matsui, T., and Takakura, N., CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment, Stem Cells, 2014, vol. 32, no. 1, pp. 145–156. https://doi.org/10.1002/stem.1556
Lo, Y.L., Sung, K.H., Chiu, C.C., and Wang, L.F., Chemically conjugating polyethylenimine with chondroitin sulfate to promoteCD44-mediated endocytosis for gene delivery, Mol. Pharm., 2013, vol. 10, no. 2, pp. 664–676. https://doi.org/10.1021/mp300432s
Dubey, R.D., Klippstein, R., Wang, J.T.-W., Hodgins, N., Mei, K.-C., Sosabowski, J., et al., Novel hyaluronic acid conjugates for dual nuclear imaging and therapy in CD44-expressing tumors in mice in vivo, Nanotheranostics, 2017, vol. 1, no. 1, pp. 59–79. https://doi.org/10.7150/ntno.17896
Guillem, V.M. and Aliño, S.F., Transfection pathways of nonspecific and targeted PEI-polyplexes, Gene Ther. Mol. Biol., 2004, vol. 8, pp. 369–384.
Ogris, M., Steinlein, P., Kursa, M., Mechtler, K., Kircheis, R., and Wagner, E., The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells, Gene Ther., 1998, vol. 5, no. 10, pp. 1425–1433. https://doi.org/10.1038/sj.gt.3300745
Wightman, L., Kircheis, R., Carotta, S., Ruzicka, R., Kursa, M., and Wagner, E., Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo, J. Gene Med., 2001, vol. 3, no. 4, pp. 362–372. https://doi.org/10.1002/jgm.187
Song, H., Wang, G., He, B., Li, L., Li, C., Lai, Y., et al., Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells, Int. J. Nanomed., 2012, vol. 7, pp. 4637–4648. https://doi.org/10.2147/IJN.S33923
Guo, W. and Lee, R.J., Efficient gene delivery via non-covalent complexes of folic acid and polyethylenimine, J. Controlled Release, 2001, vol. 77, pp. 131–138. https://doi.org/10.1016/S0168-3659(01)00456-4
Cheraghi, R., Alipour, M., Nazari, M., and Hosseinkhani, S., Optimization of conditions for gene delivery system based on PEI, Nanomed. J., 2017, vol. 4, no. 1, pp. 8–16. https://doi.org/10.22038/nmj.2017.8047
Koyama, Y., Sugiura, K., Yoshihara, C., Inaba, T., and Ito, T., Highly effective non-viral antitumor gene therapy system comprised of biocompatible small plasmid complex particles consisting of pDNA, anionic polysaccharide, and fully deprotected linear polyethylenimine, Pharmaceutics, 2015, vol. 7, no. 3, pp. 152–164. https://doi.org/10.3390/pharmaceutics7030152
Breunig, M., Lungwitz, U., Liebl, R., and Goepferich, A., Breaking up the correlation between efficacy and toxicity for nonviral gene delivery, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, no. 36, pp. 14454–14459. https://doi.org/10.1073/pnas.0703882104
Funding
This study was supported by the Russian Foundation for Basic Research, grant KOMPhI no. 17-00-00190, “The Investigation of Regulatory Elements of Specific Gene Expression in Tumor-Associated Fibroblasts and the Possibilities of their Use for Engineering of Artificial Immune Coactivator Interactions.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Novikova
About this article
Cite this article
Bulanenkova, S.S., Snezhkov, E.V., Potapov, V.K. et al. Chondroitin Sulfate Increases Transfection Efficiency by DNA–PEI Complexes. Mol. Genet. Microbiol. Virol. 34, 220–227 (2019). https://doi.org/10.3103/S0891416819040037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416819040037