Abstract
Background: Patients with schizophrenia or related disorders often switch antipsychotic therapy, most commonly due to lack of efficacy and side effects. The differences in anticipated efficacy and tolerability among atypical antipsychotics may drive switching behaviours. Switching to long-acting antipsychotics may improve adherence. Improving adherence is essential as relatively short medication gaps significantly increase the risk of schizophrenia hospitalizations. Long-term treatment with risperidone long-acting injectable (RLAI), the first available long-acting atypical antipsychotic, versus oral atypical antipsychotics showed better adherence with RLAI. Stable patients with schizophrenia or related disorders treated with a stable dose of antipsychotic showed improved efficacy when switched to flexible doses of RLAI. The most common reason for patients to switch from olanzapine to another antipsychotic is excessive weight gain. Metabolic dysfunction also occurs more commonly with olanzapine than with risperidone. Patients switching from olanzapine to risperidone experienced significant decreases in body weight, body mass index and triglyceride levels, whereas patients switching from risperidone to olanzapine experienced significant increases in body weight and triglyceride levels. The efficacy, tolerability and safety of RLAI in non-acute patients with schizophrenia or schizoaffective disorder previously treated with oral olanzapine needs to be explored.
Objective: The objective of this study was to evaluate the efficacy, tolerability and safety of switching from oral olanzapine to RLAI.
Methods: This was a six-month, prospective, multicentre, non-randomized, single-arm, open-label trial. The trial evaluated non-acute adult patients with psychotic disorders treated with a stable olanzapine dose who required a treatment change. Three weeks after RLAI initiation, olanzapine was tapered off over 1 week or 3 weeks.
Efficacy and safety measures included the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impression-Severity (CGI-S), Global Assessment of Functioning (GAF) and treatment-emergent adverse events (TEAEs).
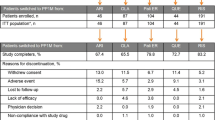
Results: Among 96 patients analysed, significant endpoint efficacy changes versus baseline were observed for PANSS, CGI-S and GAF (all p< 0.0001). PANSS total score improvement was ≥20% for 65.6% of patients and ≥50% for 31.3%. TEAEs were similar in the 1- and 3-week taper groups (40.0% and 46.5%, respectively). TEAEs were generally mild (34.5%) or moderate (49.0%) in intensity.
Conclusion: Switching non-acute patients with schizophrenia or schizoaffective disorder requiring a treatment change from a stable dose of oral olanzapine to RLAI improved psychiatric symptom control, functioning and patient treatment satisfaction. RLAI was generally well tolerated.



Similar content being viewed by others
References
Altamura AC, Armadoros D, Jaeger M, et al. Importance of open access to atypical antipsychotics for the treatment of schizophrenia and bipolar disorder: a European perspective. Curr Med Res Opin 2008; 24(8): 2271–82
Buckley PF, Correll CU. Strategies for dosing and switching antipsychotics for optimal clinical management. J Clin Psychiatry 2008; 69Suppl. 1: 4–17
Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010; (3): CD006654
Komossa K, Rummel-Kluge C, Hunger H, et al. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2009; (4): CD006627
Komossa K, Rummel-Kluge C, Schmid F, et al. Aripirprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2009; (4): CD006569
Komossa K, Rummel-Kluge C, Schmid F, et al. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010; (1): CD006625
Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2011; (1): CD006626
Law MR, Soumerai SB, Ross-Degnan D, et al. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry 2008; 69(1): 47–53
Möller HJ, Llorca PM, Sacchetti E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol 2005; 20(3): 121–30
Olivares JM, Rodriguez-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry 2009; 24(5): 287–96
Davis JM, Leucht S. Commentary on strategies for switching antipsychotics. BMC Med 2008; 6: 18
Perez-Iglesias R, Crespo-Facorro B, Amado JA, et al. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry 2007; 68(11): 1733–40
Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naïve population. Schizophr Res 2008; 99(1–3): 13–22
Saddichha S, Manjunatha N, Ameen S, et al. Effect of olanzapine, risperidone, and haloperidol treatment on weight and body mass index in first-episode schizophrenia patients in India: a randomized, double-blind, controlled, prospective study. J Clin Psychiatry 2007; 68(11): 1793–8
Su KP, Wu PL, Pariante CM. A crossover study on lipid and weight changes associated with olanzapine and risperidone. Psychopharmacology (Berl) 2005; 183(3): 383–6
Garman PM, Ried LD, Bengtson MA, et al. Effect on lipid profiles of switching from olanzapine to another second-generation antipsychotic agent in veterans with schizophrenia. J Am Pharm Assoc 2007; 47(3): 373–8
Meyer JM, Pandina G, Bossie CA, et al. Effects of switching from olanzapine to risperidone on the prevalence of the metabolic syndrome in overweight or obese patients with schizophrenia or schizoaffective disorder: analysis of a multicenter, rater-blinded, open-label study. Clin Ther 2005; 27(12): 1930–41
Ganguli R, Brar JS, Mahmoud R, et al. Assessment of strategies for switching patients from olanzapine to risperidone: a randomized, open-label, rater-blinded study. BMC Med 2008; 6: 17
American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: APA, 1994
Guy W. Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology; Rockville (MD): US Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, 1976: 218–22
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13(2): 261–76
Chouinard G, Ross-Chouinard A, Annable L, et al. Extrapyramidal Symptom Rating Scale. Can J Neurol Sci 1980; 7(3): 233
Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res 2005; 76(2–3): 247–65
Welborn TA, Dhaliwal SS. Preferred clinical measures of central obesity for predicting mortality. Eur J Clin Nutr 2007; 61(12): 1373–9
Gastpar M, Masiak M, Latif MA, et al. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol 2005; 19(5 Suppl.): 32–8
Gastpar M, Chrzanowski W, Latif MA, et al. Olanzapine to RLAI: improvements in psychotic patients [abstract]. Presented at the 8th World Congress of Biological Psychiatry (WCBP); 2005 Jun 28–Jul 3; Vienna
Csernansky JG, Mahmoud R, Brenner R, et al. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002; 346(1): 16–22
Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997; 58(12): 538–46
Gharabawi GM, Gearhart NC, Lasser RA, et al. Maintenance therapy with once-monthly administration of long-acting injectable risperidone in patients with schizophrenia or schizoaffective disorder: a pilot study of an extended dosing interval. Ann Gen Psychiatry 2007; 6: 3
Acknowledgements
The authors wish to thank Tam Vo, PhD, and the team from Excerpta Medica for provided editorial and writing assistance.
Disclosures
Funding: The study and editorial/writing support were funded by Janssen Pharmaceutical Companies of Johnson & Johnson in EMEA.
Role of funding source: Janssen Pharmaceutical Companies of Johnson & Johnson in EMEA designed the study, and was responsible for data collection. Contracted CRO was responsible for trial management, statistical planning and analyses. Editorial and writing assistance was funded by the sponsor. The authors are fully responsible for all content, editorial decisions and opinions expressed in this study. The sponsor suggested the journal for submission and all authors have agreed to the submission.
Conflicts of interest: Dr Rosa has no conflicts of interest to declare.
Dr Schreiner is a full-time employee of Janssen-Cilag Medical Affairs EMEA.
Prof. Thomas has received honoraria from: BMS–Otsuka; Janssen-Cilag; Lilly; sanofi-aventis; and Servier.
Dr Sherif has no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosa, F., Schreiner, A., Thomas, P. et al. Switching Patients with Stable Schizophrenia or Schizoaffective Disorder from Olanzapine to Risperidone Long-Acting Injectable. Clin Drug Investig 32, 267–279 (2012). https://doi.org/10.2165/11599080-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11599080-000000000-00000