Abstract
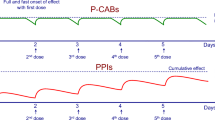
Lansoprazole is an H+, K+-adenosine triphosphatase proton pump inhibitor (PPI) used for management of acid-related disorders. Lansoprazole has been reformulated as an oro-dispersible tablet (LODT) that quickly dissolves in the mouth without water. In healthy adults the safety and bioavailability of LODT 15–30mg, taken without water or dispersed in water, were found to be comparable with those of lansoprazole 15–30mg capsules. Moreover, the bioavailability of LODT administered without water has been found to be similar to that of water-dispersed LODT given via a nasogastric tube. In a clinical study, the vast majority of patients found the mouth feel of LODT acceptable and almost all found it easy to take. A comparison of LODT with esomeprazole in a small group of patients with non-erosive reflux disease showed similar decreases in symptoms from baseline and no significant difference between groups.
In conclusion, LODT is effective, bioequivalent to the capsule formulation and acceptable to patients. LODT offers an alternative dose administration method to all patients requiring a PPI, especially those who have difficulty swallowing, and may increase patient convenience and compliance.



Similar content being viewed by others
References
Levin TR, Schmittdiel JA, Kunz K, et al. Costs of acid-related disorders to a health maintenance organization. Am J Med 1997; 103: 520–8
Scott M, Gelhot AR. Gastroesophageal reflux disease: diagnosis and management. Am Fam Physician 1999; 59: 1161–9, 1199
Locke III GR, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997; 112: 1448–56
Tolman KG, Sanders SW, Buchi KN, et al. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J Clin Gastroenterol 1997; 24: 65–70
Fitton A, Wiseman L. Pantoprazole: a review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs 1996; 51(3): 460–82
Hassan-Alin M, Andersson T, Bredberg E, et al. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol 2000; 56: 665–70
Swan SK, Hoyumpa AM, Merritt GJ. Review article: the pharmacokinetics of rabeprazole in health and disease. Aliment Pharmacol Ther 1999; 13 Suppl. 3: 11–7
Howden CW. Clinical pharmacology of omeprazole. Clin Pharmacokinet 1991; 20(1): 38–49
Stedman CAM, Barclay ML. Comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 2000; 14: 963–78
Matheson AJ, Jarvis B. Lansoprazole: an update of its place in the management of acid-related disorders. Drugs 2001; 61(12): 1801–33
Graham DY, Agrawal NM, Campbell DR, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. NSAID-Associated Gastric Ulcer Prevention Study Group. Arch Intern Med 2002; 162(2): 169–75
Misiewicz JJ, Harris AW, Bardhan KD, et al. One week triple therapy for Helicobacter pylori: a multicentre comparative study. Lansoprazole Helicobacter Study Group. Gut 1997; 41(6): 735–9
Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Physician 2002; 66(2): 273–80
Scott LJ, Dunn CJ, Mallarkey G, et al. Esomeprazole: a review of its use in the management of acid-related disorders in the US [published erratum appears in Drugs 2002; 62 (15): 2183]. Drugs 2002; 62(7): 1091–118
Tateno M, Nakamura N. Phase I study of lansoprazole antiulcer agent: capsule form. J Clin Ther Med 1991; 7: 51–62
Tabata T, Kashihara T, Hirai S, et al. Effect of gastric pH on the absorption of a new antiulcer drug (lansoprazole) in the beagle dog. J Biopharm Sci 1991; 2: 319–28
Baldi F, Malfertheiner P. Lansoprazole fast disintegrating tablet: a new formulation for an established proton pump inhibitor. Digestion 2003; 67: 1–5
Freston JW, Chiu YL, Mulford DJ, et al. Comparative pharmacokinetics and safety of lansoprazole oral capsules and orally disintegrating tablets in healthy subjects. Aliment Pharmacol Ther 2003; 17: 361–7
Gremse DA, Donnelly JR, Kukulka MJ, et al. A novel option for dosing of proton pump inhibitors: dispersion of lansoprazole orally disintegrating tablet in water via oral syringe. Aliment Pharmacol Ther 2004; 19: 1211–5
Taubel J, Lorch U, Baxter G. A comparison of the bioavailability of 15mg lansoprazole oro-dispersible tablet dispersed in the mouth with prior dispersal in water [abstract]. Gut 2003; 52 Suppl. 6: A61
Freston JW, Kukulka MJ, Lloyd E, et al. A novel option in proton pump inhibitor dosing: lansoprazole orally disintegrating tablet dispersed in water and administered via nasogastric tube. Aliment Pharmacol Ther 2004; 20: 407–11
Gerloff J, Mignot A, Barth H, et al. Pharmacokinetics and absolute bioavailability of lansoprazole. Eur J Clin Pharmacol 1996; 50: 293–7
Iwasaki K, Yoshikawa Y, Shibata N, et al. Evaluation of fast disintegrating lansoprazole tablet in human subjects. Drug Metab Pharmacokinet 2004; 19: 227–35
Pumfrey B, Baxter G. Patient administration and acceptability of the lansoprazole oro-dispersible tablet [abstract]. Gut 2004; 53 Suppl. 6: A289
Morelli P, Gambardella F, Tuccillo M, et al. Lansoprazole orally disintegrating tablets (LODT): an Italian study of awareness, use and satisfaction of this new formulation among physicians [abstract]. Digest Liver Dis 2005; 37 Suppl. 1: S81
Baldi F, Cavoli C, Ghersi S, et al. A comparison of the new lansoprazole orally disintegrating tablets (LODT) with esomeprazole in patients with non erosive reflux disease [abstract]. Digestive Disease Week; 2005 May 14–19; Chicago
Sanders SW, Tolman KG, Greski PA, et al. The effects of lansoprazole, a new H+,K(+)-ATPase inhibitor, on gastric pH and serum gastrin. Aliment Pharmacol Ther 1992; 6: 359–72
Hongo M, Ohara S, Hirasawa Y, et al. Effect of lansoprazole on intragastric pH: comparison between morning and evening dosing. Dig Dis Sci 1992; 37: 882–90
Fraser AG, Sawyerr AM, Smith MS, et al. Morning versus evening dosing of lansoprazole 30 mg daily on twenty-four-hour intragastric acidity in healthy subjects. Aliment Pharmacol Ther 1996 Aug; 10(4): 523–7
Fraser AG, Sawyerr AM, Hudson M, et al. Morning versus evening dosing of lansoprazole 30mg daily on twenty-four-hour intragastric acidity in healthy subjects. Aliment Pharmacol Ther 1996; 10: 523–7
Baldi F, Morselli-Labate AM, Cappiello R, et al. Daily low-dose versus alternate day full-dose lansoprazole in the maintenance treatment of reflux esophagitis. Am J Gastroenterol 2002; 97: 1357–64
Shanley C, O’Loughlin G. Dysphagia among nursing home residents: an assessment and management protocol. J Gerontol Nurs 2000; 26: 35–48
Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med 2000; 154: 150–4
Acknowledgements
The author thanks Takeda Chemical Industries for supporting the publication of this manuscript and for supporting the clinical study on LODT (recently presented at the Digestive Disease Week).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldi, F. Lansoprazole Oro-Dispersible Tablet. Drugs 65, 1419–1426 (2005). https://doi.org/10.2165/00003495-200565100-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565100-00007