Abstract
The field of lead-free piezoceramics, which aims to replace lead zirconate titanate (PZT) and related perovskite materials, has been vibrant for almost 15 years. Once the science in this field attained a certain stage of maturity, materials with properties better than PZT have appeared, and the first products are about to reach the marketplace. This article describes the three most promising lead-free piezoceramics currently under discussion to replace PZT. Each has a pronounced property profile geared for specific applications. Guidelines for directions for fundamental future research on as well as technology transfer to industry of lead-free piezoceramics are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European Union (EU) legislation on the restriction of hazardous substances1 as well as an article2 in Nature about 15 years ago provided the triggers for a strong effort into the science and technology of lead-free piezoceramics, with many stakeholders coming into play. While EU legislation restricts the use of lead in piezoelectric devices unless exempted under specific conditions,1 the article in Nature suggests that lead-free compositions may now be available to replace lead in certain piezoceramic applications.2 With the goal to reduce the production and waste disposal of toxic lead, research on lead-free piezoceramics strives to make lead zirconate titanate (PZT) and similar perovskite materials redundant. Shrout and Zhang3 have summarized the complexity of this task in a concise manner.
The scope of this endeavor to develop lead-free piezoceramics can be gleaned by considering the history of PZT,4,5 which highlights many years of development. The toxicity of lead, including risks to the environment during mining, processing, and disposal, is the driving force for this research. The article by Bell and Deubzer6 in this issue summarizes the current level of understanding. Legislation in Europe, and in many other parts of the world,7 to restrict and reduce hazardous substances such as lead has proven to be a strong driving force toward research into nontoxic replacements. Review processes and interactions with industry to address a variety of legislative directives have been developed for different applications. Exemption 7(c)-I, for example, has been recently reviewed and provides an exemption for lead in piezoelectronic devices until July 2021, with applications for further extension of this exemption due to the European commission by January 2020. Bell and Deubzer6 have outlined current legislation and future options.
Piezoelectricity describes the creation of an electric charge in response to a mechanical stress. The converse effect is the development of a strain as a function of an applied electric field (Figure 1). The proportionality constant is identical for both cases and is described as the piezoelectric coefficient, d.4,8 Since polarization is a vector and stress a second rank tensor, the piezoelectric constant must be written as a third rank tensor, but can be transformed to a second rank matrix.9 As we are considering the replacement of PZT and of related materials, which are ferroelectrics and are converted to piezoelectrics by a poling process, ferroelectric crystal structures are becoming important.
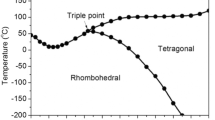
Domains in ferroelectrics (Figure 2) can be considered to be a form of twins that develop at the paraelectric/ ferroelectric phase transition in order to reduce the elastic and electrostatic energies.10 Application of an electric field may lead to lattice extension (intrinsic contribution to the piezoelectric effect), domain-wall movement resulting in a strain contribution (extrinsic contribution to the piezoelectric effect), and phase transitions.11 Consequently, the thermodynamics of the different crystal symmetries (Figure 3) is of paramount importance for understanding and designing piezoelectric materials. In particular, the anisotropic flattening of the free energy along nonpolar directions is key for good piezoelectric properties, as it facilitates easy rotation of the polarization vector.11,12 This important topic, contrasting piezoelectric mechanisms between PZT-related materials and new lead-free compositions, is covered in the article by Damjanovic and Rossetti in this issue.13
The cubic high-temperature perovskite phase transforming into one or several of the low-temperature phases: rhombohedral, orthorhombic, and tetragonal.4 Lead (barium, calcium, bismuth, potassium, or sodium in the lead-free replacements) occupies the A-site, while titanium, zirconium, niobium, tantalum or iron are on the B-site.
Note that domain wall movement, as well as phase transformations, are strongly hysteretic processes, which may lead to energy dissipation in the material. A temperature increase may then prompt thermal runaway and limit material performance. Hence, an important distinction in piezoceramics is between soft (high strain, high losses) and hard (low strain, low losses) piezoceramics.14 A further classification stems from the pertinent applied field. Piezoelectric properties under low driving field (small signal properties) and under high driving field (large signal properties) are relevant for different applications.
Lead-free material options
The endeavor to find new lead-free perovskites has mostly focused on mimicking PZT. In PZT, a so-called morphotropic phase boundary (MPB) forms and provides properties mostly independent of temperature. This MPB features changes in phase structure with just composition and not temperature. Elements with high ionic polarizability, low cost,7 and low environmental impact15 are required. Due to the similar electronic structure of bismuth and lead with the lone 2s-electron pair facilitating hybridization with oxygen, sodium-bismuthtitanate-based (NBT) materials have been contemplated since 1991.16 These materials are typically relaxors and offer complex phase diagrams.17 Consequently, dedicated studies of in situ phase transitions using scattering techniques such as neutron and synchrotron diffraction played an important role in elaborating structure–property relationships.17,18 The current understanding of lead-free relaxor-based piezoceramics is discussed in the article by Paterson et al. in this issue.19
“Lead-free at last” was the title chosen by Cross20 to accompany a paper by Saito et al.2 on the development of potassium sodium niobate (KNN)-based lead-free piezoceramics. These materials feature optimum properties at a polymorphic phase transition with attendant drawbacks of strong temperature dependence.21,22 More recent research has focused on chemical modifications to provide enhanced temperature stability of the piezoelectric properties.23 This approach and attendant materials are discussed by Wang et al.24 in their article in this issue.
Liu and Ren25 proposed an alternative developmental path to mimicking PZT in 2009, using barium titanate as the base material, doping it with calcium and zirconium, and thus furnishing it with a tricritical point25 or convergence region where the cubic, orthorhombic, tetragonal, and rhombohedral phases coexist.26 This material again features a complex phase structure with good properties, albeit limited by a low Curie temperature. In their article in this issue, Gao et al.27 discuss structure–property relationships for different compositions in the pseudobinary phase diagram Ba(Zr,Ti)O3–(Ba,Ca)TiO3. A new material class based on bismuth ferrite (BF) addresses the needs for high-temperature applications. While the first publications on bismuth ferrite-barium titanate (BF-BT) are already several years old,28 there has been recent progress by a number of researchers. Most appealing is the promise for use of BF-BT at high temperatures, not only replacing PZT, but also considerably improving high-temperature capabilities.29 In a study by Lee et al.,29 excellent piezoelectric properties with a Curie temperature in excess of 400°C were reported when BF-BT with the addition of either Bi1.05GaO3 or Bi1.05(Zn0.5Ti0.5)O3 was sintered and then quenched. Recently, a correlation of the phase diagram of BF-BT with electrical properties was achieved.30 Similarly, it was demonstrated that an air quenching treatment in BF-BT affords an improvement in piezoelectric properties through salient changes in atomic structure.31
Figure 4 summarizes the evolution in output of publications and patents in the field of lead-free piezoceramics. Work by many researchers has led to a secondary effect (i.e., development of spin-off nonpiezoelectric applications). The new insight, notably of bismuth-based perovskites, has spawned new research fields into oxygen conductors,33 high-temperature dielectrics,34 and energy-storage materials.35 Similarly, a greater understanding of niobate-based materials has yielded the development of new antiferroelectric niobates for energy storage36 and for electrocalorics.37
Evolution of lead-free piezoelectric research output in terms of (a) publications and (b) patents. This graph was compiled by searching for “lead-free” and “piezoceramics” in Web of Science32 and then checking each paper individually, whether it indeed discusses the replacement of lead zirconate titanate for piezoceramic applications. (a) The color bars represent the piezoelectric applications, while the black circles with the dashed line (lead-free perovskites, new applications) outline spin-off applications stemming from research into lead-free piezoceramics. KNN, potassium sodium niobate; NBT, sodium bismuth titanate; BT, barium titanate.
Future directions
While legislation in Europe and other regions has provided a strong impetus for further research, intense subsequent efforts have brought our scientific understanding close to the level of knowledge on lead-containing piezoceramics. This is particularly true for structural investigations of lead-free piezoceramics, where complementary in situ (temperature-, stress- and electric-field-dependent) neutron and synchrotron scattering techniques have provided a highly advanced understanding.38 As a result, avenues have opened up for new materials with properties better than PZT for select applications.39 For example, NBT-based materials have advantages over PZT in high-power applications,40 and KNN can be sintered with nickel for multilayer applications, with nickel providing high electromigration resistance and stability when subjected to high applied electric fields.41 In the area of thin films, KNN materials offer excellent properties.42 Additionally, KNN-based ceramics are preferentially used for medical imaging transducers of 1–3 structured composites (fibrous inclusions in continuous matrix).43,44 Our current knowledge of some of the primary piezoelectric properties (e.g., temperature-dependent piezoelectric properties) has reached a level sufficient for use in applications (Figure 545–90). In Figure 5, we see that BT and KNN exhibit a piezoelectric coefficient competitive to PZT at room temperature,3 while BF-BT has a higher Curie temperature than PZT.3 NBT-BT has advantages with high power applications and mechanical reliability (not covered with this graph). However, at this point in time, we also need to dedicate research efforts into better understanding secondary properties, such as electrical and mechanical properties, electrical fatigue, and machinability.91 Based on the current level of product development, the article by Shibata et al. in this issue discusses the status of product transfer into applications.92 At the same time, new materials (e.g., based on bismuth ferrite)29 and new physical mechanisms are being discovered. In terms of new mechanisms, the opportunities to enhance depolarization temperature by either quenching93 or using composites94,95 and by hardening through hard second phases96 seem particularly noteworthy.
A 3D figure showing d33 as the z-axis, and the corresponding Curie temperature/depolarization temperature (TC/Td) as the x-axis. The y-axis may be assigned to different material systems. BT and KNN exhibit a piezoelectric coefficient competitive to lead zirconate titanate (PZT) at room temperature,3 while BF-BT has a higher Curie temperature than PZT. Note: d33, piezoelectric coefficient; NBT, sodium bismuth titanate; KNN, potassium sodium niobate; B F, bismuth ferrite; BT barium titanate.2,16,25,45–90
References
EU-Directive 2002/95/EC: “Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS),” Official Journal of the European Union 46 (L37), 19 (2003).
Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya, M. Nakamura, Nature 432, 84 (2004).
T.R. Shrout, S.J. Zhang, J. Electroceram. 19, 113 (2007).
B. Jaffe, W.R. Cook, H. Jaffe, Piezoelectric Ceramics (Academic Press, Marietta, OH, 1971).
S. Trolier-McKinstry, C.A. Randall, J. Am. Ceram. Soc. 100, 3346 (2017).
A.J. Bell, O. Deubzer, MRS Bull. 43 (8), 581 (2018).
J. Rödel, W. Jo, K.T.P. Seifert, E.M. Anton, T. Granzow, D. Damjanovic, J. Am. Ceram. Soc. 92, 1153 (2009).
W. Heywang, K. Lubitz, W. Wersing, Piezoelectricity, Springer Series in Materials Science (Springer, Berlin, Germany, 2008).
R.E. Newnham, Properties of Materials: Anisotropy, Symmetry, Structure (Oxford University Press, Oxford, UK, 2005).
A.K. Tagantsev, L.E. Cross, J. Fousek, Domains in Ferroic Crystals and Thin Films (Springer, New York, 2010).
D. Damjanovic, J. Am. Ceram. Soc. 88, 2662 (2005).
G.A. Rossetti, A.G. Khachaturyan, G. Akcay, Y. Ni, J. Appl. Phys. 103, 114113 (2008).
D. Damjanovic, G.A. Rossetti Jr., MRS Bull. 43 (8), 588 (2018).
K. Uchino, Ferroelectric Devices (Marcel Dekker, New York, 2000).
T. Ibn-Mohammed, S.C.L. Koh, I.M. Reaney, A. Acquaye, D. Wang, S. Taylor, A. Genovese, Energy Environ. Sci. 9, 3495 (2016).
T. Takenaka, K. Maruyama, K. Sakata, Jpn. J. Appl. Phys. Pt. 1 30, 2236 (1991).
C. Ma, H. Guo, S.P. Beckman, X. Tan, Phys. Rev. Lett. 109, 107602 (2012).
J.E. Daniels, W. Jo, J. Rödel, J.L. Jones, Appl. Phys. Lett. 95, 032904 (2009).
A.R. Paterson, H. Nagata, X. Tan, J.E. Daniels, M. Hinterstein, R. Ranjan, P.B. Groszewicz, W. Jo, J.L. Jones, MRS Bull. 43 (8), 600 (2018).
L.E. Cross, Nature 432, 24 (2004).
E. Hollenstein, D. Damjanovic, N. Setter, J. Eur. Ceram. Soc. 27, 4093 (2007).
S.J. Zhang, R. Xia, T.R. Shrout, Appl. Phys. Lett. 91, 132913 (2007).
R. Wang, H. Bando, T. Katsumata, Y. Inaguma, H. Taniguchi, M. Itoh, Phys. Status Solidi Rapid Res. Lett. 3, 142 (2009).
K. Wang, B. Malič, J. Wu, MRS Bull. 43 (8), 607 (2018).
W.F. Liu, X.B. Ren, Phys. Rev. Lett. 103, 257602 (2009).
D.S. Keeble, F. Benabdallah, P.A. Thomas, M. Maglione, J. Kreisel, Appl. Phys. Lett. 102, 092903 (2013).
J. Gao, X. Ke, M. Acosta, J. Glaum, X. Ren, MRS Bull. 43 (8), 595 (2018).
S.O. Leontsev, R.E. Eitel, J. Mater. Res. 26, 9 (2011).
M.H. Lee, D.J. Kim, J.S. Park, S.W. Kim, T.K. Song, M.H. Kim, W.J. Kim, D. Do, I.K. Jeong, Adv. Mater. 27, 6976 (2015).
S. Kim, G.P. Khanal, H.-W. Nam, J. Fujii, S. Ueno, C. Moriyoshi, Y. Kuroiwa, S. Wada, J. Appl. Phys. 122, 164105 (2017).
I. Calisir, D.A. Hall, J. Mater. Chem. C 6, 134 (2018).
https://clarivate.com/products/web-of-scienceproducts/web-of-science.
M. Li, M.J. Pietrowski, R.A. De Souza, H. Zhang, I.M. Reaney, S.N. Cook, J.A. Kilner, D.C. Sinclair, Nat. Mater. 13, 31 (2014).
R. Dittmer, W. Jo, D. Damjanovic, J. Rödel, J. Appl. Phys. 109, 034107 (2011).
F. Gao, X. Dong, C. Mao, W. Liu, H. Zhang, L. Yang, F. Cao, G. Wang, J. Am. Ceram. Soc. 94, 4382 (2011).
H. Guo, H. Shimizu, Y. Mizuno, C.A. Randall, J. Appl. Phys. 117, 214103 (2015).
J. Koruza, B. Rožič, G. Cordoyiannis, B. Malič, Z. Kutnjak, Appl. Phys. Lett. 106, 202905 (2015).
I. Levin, I.M. Reaney, Adv. Funct. Mater. 22, 3445 (2012).
J. Rödel, K.G. Webber, R. Dittmer, W. Jo, M. Kimura, D. Damjanovic, J. Eur. Ceram. Soc. 35, 1659 (2015).
Y. Doshida, H. Shimizu, Y. Mizuno, H. Tamura, Jpn. J. Appl. Phys. 52, 07HE01 (2013).
S. Kawada, M. Kimura, Y. Higuchi, H. Takagi, Appl. Phys. Express 2, 111401 (2009).
K. Shibata, K. Suenaga, A. Nomoto, T. Mishima, Jpn. J. Appl. Phys. 48, 121408 (2009).
Z.-Y. Shen, J.-F. Li, R.M. Chen, Q. Zhou, K.K. Shung, J. Am. Ceram. Soc. 94 (5), 1346 (2011).
J.-F. Li, K. Wang, F.-Y. Zhu, L.-Q. Cheng, F.-Z. Yao, J. Am. Ceram. Soc. 96 (12), 3677 (2013).
B. Wu, H. Wu, J. Wu, D. Xiao, J. Zhu, S.J. Pennycook, J. Am. Chem. Soc. 138, 15459 (2016).
C. Peng, J.-F. Li, W. Gong, Mater Lett. 59, 1576 (2005).
Q. Zheng, L. Luo, K.H. Lam, N. Jiang, Y. Guo, D. Lin, J. Appl. Phys. 116 (18), 184101 (2014).
Q. Zhou, C. Zhou, H. Yang, G. Chen, W. Li, H. Wang, J. Am. Ceram. Soc. 95 (12), 3889 (2012).
S.O. Leontsev, R.E. Eitel, J. Am. Ceram. Soc. 92, 2957 (2009).
T. Zheng, H. Wu, Y. Yuan, X. Lv, Q. Li, T. Men, C. Zhao, D. Xiao, J. Wu, K. Wang, J.-F. Li, Y. Gu, J. Zhu, S.J. Pennycook, Energy Environ. Sci. 10, 528 (2017).
W. Jo, J.E. Daniels, J.L. Jones, X. Tan, P.A. Thomas, D. Damjanovic, J. Rödel, J. Appl. Phys. 109, 014110 (2011).
W. Li, Z. Xu, R. Chu, P. Fu, G. Zang, Mat. Sci. Eng. B 176, 65 (2011).
W. Li, Z. Xu, R. Chu, P. Fu, P. An, Ceram. Int. 38, 4353 (2012).
W. Li, Z. Xu, R. Chu, P. Fu, G. Zang, J. Am. Ceram. Soc. 94, 4131 (2011).
X. Wang, J. Wu, D. Xiao, J. Zhu, X. Cheng, T. Zheng, B. Zhang, X. Lou, X. Wang, J. Am. Chem. Soc. 136, 2905 (2014).
X.X. Wang, X.G. Tang, H.L.W. Chan, Appl. Phys. Lett. 85, 91 (2004).
Y. Huang, L. Gao, Y. Hu, H. Du, J. Mater. Sci. Mater. Electron. 18, 605 (2007).
Y.-J. Dai, S.-S. He, X. Lao, S.-Z. Zhang, J. Am. Ceram. Soc. 97, 1283 (2014).
Y. Chang, Z.-P. Yang, D. Ma, Z. Liu, Z. Wang, J. Appl. Phys. 104, 024109 (2008).
L. Gao, Y. Huang, L. Liu, T. Liu, C. Liu, F. Zhou, X. Wan, J. Mater. Sci. 43, 6267 (2008).
A. Hussain, C.W. Ahn, J.S. Lee, A. Ullah, I.W. Kim, Sens. Actuators A Phys. 158, 84, (2010).
C. Ye, J. Hao, B. Shen, J. Zhai, J. Am. Ceram. Soc. 95, 3577 (2012).
C. Zhou, A. Feteira, X. Shan, H. Yang, Q. Zhou, J. Cheng, W. Li, H. Wang, Appl. Phys. Lett. 101, 032901 (2012).
D. Maurya, Y. Zhou, Y. Wang, Y. Yan, J. Li, D. Viehland, S. Priya, Sci. Rep. 5, 8595 (2015).
D.-S. Yin, Z.-H. Zhao, Y.-J. Dai, Z. Zhao, X.-W. Zhang, S.-H. Wang, J. Am. Ceram. Soc. 99, 2354 (2016).
F. Wang, M. Xu, Y. Tang, T. Wang, W. Shi, C.M. Leung, J. Am. Ceram. Soc. 95, 1955 (2012).
J. Wu, T. Peng, Y. Wang, D. Xiao, J. Zhu, Y. Jin, J. Zhu, P. Yu, L. Wu, Y. Jiang, J. Am. Ceram. Soc. 91, 319 (2008).
J. Wu, Y. Wang, D. Xiao, J. Zhu, P. Yu, L. Wu, W. Wu, Jpn. J. Appl. Phys. 46, 7375 (2007).
K. Wang, J.F. Li, N. Liu, Appl. Phys. Lett. 93 (9), 092904 (2008).
K. Xu, J. Li, X. Lv, J. Wu, X. Zhang, D. Xiao, J. Zhu, Adv. Mater. 28, 8519 (2016).
L. Zhu, B. Zhang, L. Zhao, J. Li, J. Mater. Chem. C 2, 4764 (2014).
M. Naderer, T. Kainz, D. Schütz, K. Reichmann, J. Eur. Ceram. Soc. 34, 663 (2014).
P.-Y. Chen, C.-S. Chen, C.-S. Tu, T.-L. Chang, J. Eur. Ceram. Soc. 34, 4223 (2014).
Q. Liu, F.-Y. Zhu, L. Zhao, K. Wang, L. Li, J.-F. Li, W. Jo, J. Am. Ceram. Soc. 99, 3670 (2016).
W. Bai, L. Li, W. Li, B. Shen, J. Zhai, H. Chen, J. Alloys Compd. 603, 149 (2014).
W. Jo, R. Dittmer, M. Acosta, J. Zang, C. Groh, E. Sapper, K. Wang, J. Rödel, J. Electroceram. 29, 71 (2012).
W. Jo, J.-B. Ollagnier, J.-L. Park, E.-M. Anton, O.J. Kwon, C. Park, H.-H. Seo, J.-S. Lee, E. Erdem, R.-A. Eichel, J. Rödel, J. Eur. Ceram. Soc. 31, 2107 (2011).
W. Krauss, D. Schütz, F. Mautner, J. Eur. Ceram. Soc. 30, 1827 (2010).
W. Li, X. Liu, J. Ma, Y. Wu, Y. Cui, J. Mater. Sci. Mater. Electron. 24, 1551 (2012).
W. Li, Z. Xu, R. Chu, P. Fu, G. Zang, M. Narayanan, J. Am. Ceram. Soc. 94, 3181 (2011).
X. Li, J. Zhu, M. Wang, Y. Luo, W. Shi, L. Li, J. Zhu, D.J. Xiao, J. Alloys Compd. 499, L1 (2010).
X. Zhou, C. Zhou, Q. Zhou, H. Yang, Z. Cen, J. Cheng, L. Cao, Q. Fan, J. Electron. Mater. 43 (3), 755 (2014).
Y. Hiruma, Y. Imai, Y. Watanabe, H. Nagata, T. Takenaka, Appl. Phys. Lett. 92, 262904 (2008).
Y. Hiruma, H. Nagata, T. Takenaka, J. Appl. Phys. 105, 084112 (2009).
Y. Sung, J. Kim, J.H. Cho, T.K. Song, M.H. Kim, T.G. Park, Appl. Phys. Lett. 98, 012902 (2011).
Y. Zhang, J. Glaum, C. Groh, M.C. Ehmke, J.E. Blendell, K.J. Bowman, M.J. Hoffman, J. Am. Ceram. Soc. 97 (9), 2885 (2014).
Y.-R. Zhang, J.-F. Li, B.-P. Zhang, C.-E. Peng, J. Appl. Phys. 103, 074109 (2008).
Z. Cen, C. Zhou, J. Cheng, X. Zhou, W. Li, C. Yan, S. Feng, Y. Liu, D. Lao, J. Alloys Compd. 567, 110 (2013).
Z. Cen, C. Zhou, H. Yang, Qin Zhou, W. Li, C. Yan, L. Cao, J. Song, L. Peng, J. Am. Ceram. Soc. 96 (7), 2252 (2013).
Z. Yang, B. Liu, L. Wei, Y. Hou, Mater. Res. Bull. 43, 81 (2008).
J. Koruza, A.J. Bell, T. Frömling, K.G. Webber, K. Wang, J. Rödel, J. Materiomics 4, 13 (2018).
K. Shibata, R. Wang, T. Tou, J. Koruza, MRS Bull. 43 (8), 612 (2018).
T. Mira, H. Nagata, T. Takenaka, Jpn. J. Appl. Phys. 56, 10PD05 (2017).
J. Zhang, Z. Pan, F.-F. Guo, W.-C. Liu, H. Ning, Y.B. Chen, M.-H. Lu, B. Yang, J. Chen, S.-T. Zhang, X. Xing, J. Rödel, W. Cao, Y.-F. Chen, Nat. Commun. 6, 6615 (2015).
L.M. Riemer, L.K. Venkataraman, X. Jiang, N. Liu, C. Dietz, R. Stark, P.B. Groszewicz, G. Buntkowsky, J. Chen, S.-T. Zhang, J. Rödel, J. Koruza, Acta Mater. 136, 271 (2017).
K.V. Lalitha, L.M. Riemer, J. Koruza, J. Rödel, Appl. Phys. Lett. 111, 022905 (2017).
Acknowledgments
We thank J. Koruza for valuable discussions, and Y. Zhang and F. Weyland for their assistance in figure preparation. J.F.L. thanks the NSFC for support under Grant Nos. 51332002 and 51761135118.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rödel, J., Li, JF. Lead-free piezoceramics: Status and perspectives. MRS Bulletin 43, 576–580 (2018). https://doi.org/10.1557/mrs.2018.181
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2018.181