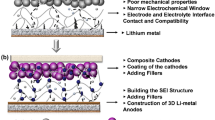
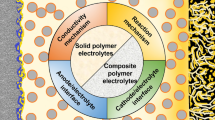
Abstract
Solid polymer electrolytes are a crucial class of compounds in the next-generation solid-state lithium batteries featured by high safety and extraordinary energy density. This review highlights the importance of carbonyl-coordinating polymer-based solid polymer electrolytes in next-generation safe and high–energy density lithium metal batteries, unraveling their synthesis, sustainability, and electrochemical performance.With the massive consumption of fossil fuel in vehicles nowadays, the resulted air pollution and greenhouse gases issue have now aroused the global interest on the replacement of the internal combustion engines with engine systems using renewable energy. Thus, the commercial electric vehicle market is growing fast. As the requirement for longer driving distances and higher safety in commercial electric vehicles becomes more demanding, great endeavors have been devoted to developing the next-generation solid-state lithium metal batteries using high-voltage cathode materials, e.g., high nickel (Ni) ternary active materials, LiCoO2, and spinel LiNi0.5Mn1.5O4. However, the most extensively investigated solid polymer electrolytes (SPEs) are based on polyether-based polymers, especially the archetypal poly(ethylene oxide), which are still suffering from low ionic conductivity (10−7 to 10−6 S/cm at room temperature), limited lithium ion transference number (<0.2), and narrow electrochemical stability window (<3.9 V), restricting this type of SPEs from realizing their full potential for the next-generation lithium-based energy storage technologies. As a promising class of alternative polymer hosts for SPEs, carbonyl-coordinating polymers have been extensively researched, exhibiting unique and promising electrochemical properties. Herein, the synthesis, sustainability, and electrochemical performance of carbonyl-coordinating SPEs for high-voltage solid-state lithium batteries will be reviewed.

















Similar content being viewed by others
Change history
01 July 2020
An Erratum to this paper has been published: https://doi.org/10.1557/mre.2020.17
References
Mohanty D., Li J., Nagpure S.C., Wood D.L., and Daniel C.: Understanding the structure and structural degradation mechanisms in high-voltage, lithium-manganese–rich lithium-ion battery cathode oxides: A review of materials diagnostics. MRS Energy Sustainability 2, E15 (2015).
US Energy Information Administration (2018). Available at: http://www.eia.gov/ (accessed February 2020).
Lewis N.S.: Powering the planet. MRS Bull. 32, 808 (2007).
Goodenough J.B. and Kim Y.: Challenges for rechargeable Li batteries. Chem. Mater. 22, 587 (2010).
Bresser D., Hosoi K., Howell D., Li H., Zeisel H., Amine K., and Passerini S.: Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Sources 382, 176 (2018).
Tarascon J.M. and Armand M.: Issues and challenges facing rechargeable lithium batteries. Nature 414, 359 (2001).
Quartarone E. and Mustarelli P.: Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 40, 2525 (2011).
Goodenough J.B. and Park K.-S.: The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 135, 1167 (2013).
Goodenough J.B. and Singh P.: Review—Solid electrolytes in rechargeable electrochemical cells. J. Electrochem. Soc. 162, A2387 (2015).
Goodenough J.B.: How we made the Li-ion rechargeable battery. Nat. Electron. 1, 204 (2018).
Manthiram A., Yu X., and Wang S.: Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Osada I., de Vries H., Scrosati B., and Passerini S.: Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem., Int. Ed. 55, 500 (2016).
Sarabi S., Kefsi L., Merdassi A., and Robyns B.: Supervision of plug-in electric vehicles connected to the electric distribution grids. Int. J. Electr. Energy 1, 256 (2013).
Wright P.V.: Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 7, 319 (1975).
Armand M.: Polymer solid electrolytes—An overview. Solid State Ionics 9–10, 745 (1983).
Wang C., Zhang H., Li J., Chai J., Dong S., and Cui G.: The interfacial evolution between polycarbonate-based polymer electrolyte and Li-metal anode. J. Power Sources 397, 157 (2018).
Fish D. and Smid J.: Solvation of lithium ions in mixtures of tetraethylene glycol dimethyl ether and propylene carbonate. Electrochim. Acta 37, 2043 (1992).
Zhang C., Ueno K., Yamazaki A., Yoshida K., Moon H., Mandai T., Umebayashi Y., Dokko K., and Watanabe M.: Chelate effects in glyme/lithium bis(trifluoromethanesulfonyl)amide solvate ionic liquids. I. Stability of solvate cations and correlation with electrolyte properties. J. Phys. Chem. B 118, 5144 (2014).
Wu J., Rao Z., Cheng Z., Yuan L., Li Z., and Huang Y.: Ultrathin, flexible polymer electrolyte for cost-effective fabrication of all-solid-state lithium metal batteries. Adv. Energy Mater. 9, 1902767 (2019).
Xu C., Sun B., Gustafsson T., Edström K., Brandell D., and Hahlin M.: Interface layer formation in solid polymer electrolyte lithium batteries: An XPS study. J. Mater. Chem. A 2, 7256 (2014).
Wei Z., Chen S., Wang J., Wang Z., Zhang Z., Yao X., Deng Y., and Xu X.: Superior lithium ion conduction of polymer electrolyte with comb-like structure via solvent-free copolymerization for bipolar all-solid-state lithium battery. J. Mater. Chem. A 6, 13438 (2018).
Di Noto V., Lavina S., Giffin G.A., Negro E., and Scrosati B.: Polymer electrolytes: Present, past and future. Electrochim. Acta 57, 4 (2011).
Meyer W.H.: Polymer electrolytes for lithium-ion batteries. Adv. Mater. 10, 439 (1998).
Agrawal R.C. and Pandey G.P.: Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. J. Phys. D: Appl. Phys. 41, 223001 (2008).
Fergus J.W.: Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 195, 4554 (2010).
Hallinan D.T. and Balsara N.P.: Polymer electrolytes. Annu. Rev. Mater. Res. 43, 503 (2013).
Xue Z., He D., and Xie X.: Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 3, 19218 (2015).
Mindemark J., Lacey M.J., Bowden T., and Brandell D.: Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114 (2018).
Zhang J., Yang J., Dong T., Zhang M., Chai J., Dong S., Wu T., Zhou X., and Cui G.: Aliphatic polycarbonate-based solid-state polymer electrolytes for advanced lithium batteries: Advances and perspective. Small 14, 1800821 (2018).
Manuel Stephan A.: Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 42, 21 (2006).
Xu K.: Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303 (2004).
Druger S.D., Nitzan A., and Ratner M.A.: Dynamic bond percolation theory: A microscopic model for diffusion in dynamically disordered systems. I. Definition and one-dimensional case. J. Chem. Phys. 79, 3133 (1983).
Webb M.A., Savoie B.M., Wang Z.-G., and Miller T.F. III: Chemically specific dynamic bond percolation model for ion transport in polymer electrolytes. Macromolecules 48, 7346 (2015).
Song J.Y., Wang Y.Y., and Wan C.C.: Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 77, 183 (1999).
Wang C., Wang T., Wang L., Hu Z., Cui Z., Li J., Dong S., Zhou X., and Cui G.: Differentiated lithium salt design for multilayered PEO electrolyte enables a high-voltage solid-state lithium metal battery. Adv. Sci. 6, 1901036 (2019).
Xu K.: Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503 (2014).
Zhou Q., Ma J., Dong S., Li X., and Cui G.: Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater. 31, e1902029 (2019).
Zaheer M., Xu H., Wang B., Li L., and Deng Y.: An in situ polymerized comb-like PLA/PEG-based solid polymer electrolyte for lithium metal batteries. J. Electrochem. Soc. 167, 070504 (2020).
Takahashi Y. and Tadokoro H.: Structural studies of polyethers, (–(CH2)m–O–)n. X. Crystal structure of poly(ethylene oxide). Macromolecules 6, 672 (1973).
Gadjourova Z., Andreev Y.G., Tunstall D.P., and Bruce P.G.: Ionic conductivity in crystalline polymer electrolytes. Nature 412, 520 (2001).
Cheng S., Smith D.M., and Li C.Y.: How does nanoscale crystalline structure affect ion transport in solid polymer electrolytes? Macromolecules 47, 3978 (2014).
Zhou Q., Zhang J., and Cui G.: Rigid–flexible coupling polymer electrolytes toward high-energy lithium batteries. Macromol. Mater. Eng. 303, 1800337 (2018).
Matsubara K., Kaneuchi R., and Maekita N.: 13C NMR estimation of preferential solvation of lithium ions in non-aqueous mixed solvents. J. Chem. Soc., Faraday Trans. 94, 3601 (1998).
Ong M.T., Verners O., Draeger E.W., van Duin A.C.T., Lordi V., and Pask J.E.: Lithium ion solvation and diffusion in bulk organic electrolytes from first-principles and classical reactive molecular dynamics. J. Phys. Chem. B 119, 1535 (2015).
Bogle X., Vazquez R., Greenbaum S., Cresce A.V.W., and Xu K.: Understanding Li+–solvent interaction in nonaqueous carbonate electrolytes with 17O NMR. J. Phys. Chem. Lett. 4, 1664 (2013).
Tominaga Y. and Yamazaki K.: Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem. Commun. 50, 4448 (2014).
Kimura K., Motomatsu J., and Tominaga Y.: Correlation between solvation structure and ion-conductive behavior of concentrated poly(ethylene carbonate)-based electrolytes. J. Phys. Chem. C 120, 12385 (2016).
Okumura T. and Nishimura S.: Lithium ion conductive properties of aliphatic polycarbonate. Solid State Ionics 267, 68 (2014).
Doyle M., Fuller T.F., and Newman J.: The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 39, 2073 (1994).
Thomas K.E., Sloop S.E., Kerr J.B., and Newman J.: Comparison of lithium-polymer cell performance with unity and nonunity transference numbers. J. Power Sources 89, 132 (2000).
Doyle M. and Newman J.: The use of mathematical modeling in the design of lithium/polymer battery systems. Electrochim. Acta 40, 2191 (1995).
Brissot C., Rosso M., Chazalviel J.N., and Lascaud S.: Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81–82, 925 (1999).
Gorecki W., Jeannin M., Belorizky E., Roux C., and Armand M.: Physical properties of solid polymer electrolyte PEO(LiTFSI) complexes. J. Phys.: Condens. Matter 7, 6823 (1995).
Borodin O. and Smith G.D.: Mechanism of ion transport in amorphous poly(ethylene oxide)/LiTFSI from molecular dynamics simulations. Macromolecules 39, 1620 (2006).
Mao G., Saboungi M.-L., Price D.L., Armand M.B., and Howells W.S.: Structure of liquid PEO-LiTFSI electrolyte. Phys. Rev. Lett. 84, 5536 (2000).
Kim C.S. and Oh S.M.: Importance of donor number in determining solvating ability of polymers and transport properties in gel-type polymer electrolytes. Electrochim. Acta 45, 2101 (2000).
Chen L., Venkatram S., Kim C., Batra R., Chandrasekaran A., and Ramprasad R.: Electrochemical stability window of polymeric electrolytes. Chem. Mater. 31, 4598 (2019).
Mindemark J., Sun B., Törmä E., and Brandell D.: High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature. J. Power Sources 298, 166 (2015).
Manuel Stephan A. and Nahm K.S.: Review on composite polymer electrolytes for lithium batteries. Polymer 47, 5952 (2006).
Sun C., Liu J., Gong Y., Wilkinson D.P., and Zhang J.: Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33, 363 (2017).
Cheng X.B., Hou T.Z., Zhang R., Peng H.J., Zhao C.Z., Huang J.Q., and Zhang Q.: Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 28, 2888 (2016).
Yang Q., Li W., Dong C., Ma Y., Yin Y., Wu Q., Xu Z., Ma W., Fan C., and Sun K.: PIM-1 as an artificial solid electrolyte interphase for stable lithium metal anode in high-performance batteries. J. Energy Chem. 42, 83 (2020).
Sun B., Mindemark J., Edstrom K., and Brandell D.: Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ionics 262, 738 (2014).
Silva M.M., Barros S.C., Smith M.J., and MacCallum J.R.: Characterization of solid polymer electrolytes based on poly(trimethylenecarbonate) and lithium tetrafluoroborate. Electrochim. Acta 49, 1887 (2004).
Barbosa P.C., Rodrigues L.C., Silva M.M., and Smith M.J.: Characterization of pTMCnLiPF6 solid polymer electrolytes. Solid State Ionics 193, 39 (2011).
Kobayashi S.: Enzymatic ring-opening polymerization and polycondensation for the green synthesis of polyesters. Polym. Adv. Technol. 26, 677 (2015).
Artham T. and Doble M.: Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 8, 14 (2008).
Cameron D.J.A. and Shaver M.P.: Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 40, 1761 (2011).
Brannigan R.P. and Dove A.P.: Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 5, 9 (2017).
Dai Y. and Zhang X.: Recent development of functional aliphatic polycarbonates for the construction of amphiphilic polymers. Polym. Chem. 8, 7429 (2017).
Hussain T., Tausif M., and Ashraf M.: A review of progress in the dyeing of eco-friendly aliphatic polyester-based polylactic acid fabrics. J. Clean. Prod. 108, 476 (2015).
Yu Y., Wu D., Liu C., Zhao Z., Yang Y., and Li Q.: Lipase/esterase-catalyzed synthesis of aliphatic polyesters via polycondensation: A review. Process Biochem. 47, 1027 (2012).
Malmstroem E., Johansson M., and Hult A.: Hyperbranched aliphatic polyesters. Macromolecules 28, 1698 (1995).
Undin J., Plikk P., Finne-Wistrand A., and Albertsson A.-C.: Synthesis of amorphous aliphatic polyester-ether homo- and copolymers by radical polymerization of ketene acetals. J. Polym. Sci., Part A: Polym. Chem. 48, 4965 (2010).
Mehta R., Kumar V., Bhunia H., and Upadhyay S.N.: Synthesis of poly(lactic acid): A review. J. Macromol. Sci. Part C: Polym. Rev. 45, 325 (2005).
Jiang Z.: Lipase-catalyzed synthesis of aliphatic polyesters via copolymerization of lactone, dialkyl diester, and diol. Biomacromolecules 9, 3246 (2008).
Varma I.K., Albertsson A.-C., Rajkhowa R., and Srivastava R.K.: Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 30, 949 (2005).
Zhang J., Shi H., Wu D., Xing Z., Zhang A., Yang Y., and Li Q.: Recent developments in lipase-catalyzed synthesis of polymeric materials. Process Biochem. 49, 797 (2014).
Douka A., Vouyiouka S., Papaspyridi L.-M., and Papaspyrides C.D.: A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 79, 1 (2018).
Williams C.K.: Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 36, 1573 (2007).
Liu Z.-L., Zhou Y., and Zhuo R.-X.: Synthesis and properties of functional aliphatic polycarbonates. J. Polym. Sci., Part A: Polym. Chem. 41, 4001 (2003).
Wang X.-L., Zhuo R.-X., Liu L.-J., He F., and Liu G.: Synthesis and characterization of novel aliphatic polycarbonates. J. Polym. Sci., Part A: Polym. Chem. 40, 70 (2002).
Tempelaar S., Mespouille L., Coulembier O., Dubois P., and Dove A.P.: Synthesis and post-polymerisation modifications of aliphatic poly(carbonate)s prepared by ring-opening polymerisation. Chem. Soc. Rev. 42, 1312 (2013).
Gross R., Kalra B., and Kumar A.: Polyester and polycarbonate synthesis by in vitro enzyme catalysis. Appl. Microbiol. Biotechnol. 55, 655 (2001).
Taherimehr M. and Pescarmona P.P.: Green polycarbonates prepared by the copolymerization of CO2 with epoxides. J. Appl. Polym. Sci. 131, 41141 (2014).
Tamura M., Ito K., Honda M., Nakagawa Y., Sugimoto H., and Tomishige K.: Direct copolymerization of CO2 and diols. Sci. Rep. 6, 24038 (2016).
Carothers W.H., Dorough G.L., and Natta F.J.v.: Studies of polymerization and ring formation. X. The reversible polymerization of six-membered cyclic esters. J. Am. Chem. Soc. 54, 761 (1932).
Bendler J.T.: Handbook of Polycarbonate Science and Technology, 1st ed. (CRC Press, New York, 1999).
Zhu W., Huang X., Li C., Xiao Y., Zhang D., and Guan G.: High-molecular-weight aliphatic polycarbonates by melt polycondensation of dimethyl carbonate and aliphatic diols: Synthesis and characterization. Polym. Int. 60, 1060 (2011).
Park J.H., Jeon J.Y., Lee J.J., Jang Y., Varghese J.K., and Lee B.Y.: Preparation of high-molecular-weight aliphatic polycarbonates by condensation polymerization of diols and dimethyl carbonate. Macromolecules 46, 3301 (2013).
Mespouille L., Coulembier O., Kawalec M., Dove A.P., and Dubois P.: Implementation of metal-free ring-opening polymerization in the preparation of aliphatic polycarbonate materials. Prog. Polym. Sci. 39, 1144 (2014).
Möller M., Hedrick J.L., Degée P., and Dubois P.: Ring opening polymerization. In Encyclopedia of Materials: Science and Technology, Buschow K.H.J., Cahn R.W., Flemings M.C., Ilschner B., Kramer E.J., Mahajan S. and Veyssière P., eds. (Elsevier, Oxford, 2001); p. 8202.
Jérôme C. and Lecomte P.: Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Delivery Rev. 60, 1056 (2008).
Paul S., Zhu Y., Romain C., Brooks R., Saini P.K., and Williams C.K.: Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 51, 6459 (2015).
Xu J., Feng E., and Song J.: Renaissance of aliphatic polycarbonates: New techniques and biomedical applications. J. Appl. Polym. Sci. 131, 39822 (2014).
D’Alessandro D.M., Smit B., and Long J.R.: Carbon dioxide capture: Prospects for new materials. Angew. Chem., Int. Ed. 49, 6058 (2010).
Fukuoka S., Kawamura M., Komiya K., Tojo M., Hachiya H., Hasegawa K., Aminaka M., Okamoto H., Fukawa I., and Konno S.: A novel non-phosgene polycarbonate production process using by-product CO2 as starting material. Green Chem. 5, 497 (2003).
Darensbourg D.J., Mackiewicz R.M., Phelps A.L., and Billodeaux D.R.: Copolymerization of CO2 and epoxides catalyzed by metal salen complexes. Acc. Chem. Res. 37, 836 (2004).
Sugimoto H. and Inoue S.: Copolymerization of carbon dioxide and epoxide. J. Polym. Sci., Part A: Polym. Chem. 42, 5561 (2004).
Inoue S., Koinuma H., and Tsuruta T.: Copolymerization of carbon dioxide and epoxide. J. Polym. Sci., Part B: Polym. Lett. 7, 287 (1969).
Lu X.-B., Ren W.-M., and Wu G.-P.: CO2 copolymers from epoxides: Catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 45, 1721 (2012).
Coates G.W. and Moore D.R.: Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem., Int. Ed. 43, 6618 (2004).
Albertsson A.-C. and Varma I.K.: Aliphatic Polyesters: Synthesis, Properties and Applications, Degradable Aliphatic Polyesters (Springer Berlin Heidelberg, Berlin, Heidelberg, 2002); p. 1.
Park E.-S., Cho H.-C., Kim M.-N., and Yoon J.-S.: Chain extension and mechanical properties of unsaturated aliphatic copolyesters based on poly(L-lactic acid). J. Appl. Polym. Sci. 90, 1802 (2003).
Eyvazzadeh Kalajahi A., Rezaei M., Abbasi F., and Mir Mohamad Sadeghi G.: The effect of chain extender type on the physical, mechanical, and shape memory properties of poly(ε-caprolactone)-based polyurethane-ureas. Polym. Plast. Technol. Eng. 56, 1977 (2017).
Zhao J.-B., Wu X.-F., and Yang W.-T.: Synthesis of aliphatic polyesters by a chain-extending reaction with octamethylcyclotetrasilazane and hexaphenylcyclotrisilazane as chain extenders. J. Appl. Polym. Sci. 92, 3333 (2004).
Löfgren A., Albertsson A.-C., Dubois P., and Jérôme R.: Recent advances in ring-opening polymerization of lactones and related compounds. J. Macromol. Sci. Part C: Polym. Rev. 35, 379 (1995).
Webb A.R., Yang J., and Ameer G.A.: Biodegradable polyester elastomers in tissue engineering. Expert Opin. Biol. Ther. 4, 801 (2004).
Tokiwa Y. and Calabia B.P.: Review degradation of microbial polyesters. Biotechnol. Lett. 26, 1181 (2004).
Silvers A.L., Chang C.-C., Parrish B., and Emrick T.: Strategies in Aliphatic Polyester Synthesis for Biomaterial and Drug Delivery Applications, Degradable Polymers and Materials: Principles and Practice, 2nd ed. (American Chemical Society, Washington D.C., 2012); p. 237.
Hakkarainen M.: Aliphatic Polyesters: Abiotic and Biotic Degradation and Degradation Products (Degradable Aliphatic Polyesters, Springer, Berlin, Heidelberg, 2002); p. 113.
Hilf J. and Frey H.: Propargyl-functional aliphatic polycarbonate obtained from carbon dioxide and glycidyl propargyl ether. Macromol. Rapid Commun. 34, 1395 (2013).
Liu F., Yang J., Fan Z., Li S., Kasperczyk J., and Dobrzynski P.: Enzyme-catalyzed degradation of biodegradable polymers derived from trimethylene carbonate and glycolide by lipases from Candida Antarctica and Hog pancreas. J. Biomater. Sci. Polym. Ed. 23, 1355 (2012).
Kaplan M.L., Rietman E.A., Cava R.J., Holt L.K., and Chandross E.A.: Crown ether enhancement of ionic conductivity in a polymer-salt system. Solid State Ionics 25, 37 (1987).
Wei X. and Shriver D.F.: Highly conductive polymer electrolytes containing rigid polymers. Chem. Mater. 10, 2307 (1998).
Matsumoto K., Kakehashi M., Ouchi H., Yuasa M., and Endo T.: Synthesis and properties of polycarbosilanes having 5-membered cyclic carbonate groups as solid polymer electrolytes. Macromolecules 49, 9441 (2016).
Chai J., Liu Z., Ma J., Wang J., Liu X., Liu H., Zhang J., Cui G., and Chen L.: In situ generation of poly(vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 4, 1600377 (2017).
Xu H., Bijleveld J., Hedge M., and Dingemans T.: Synthesis and characterization of aromatic-PDMS segmented block copolymers and their shape-memory performance. Polym. Chem. 10, 5052 (2019).
Soo P.P., Huang B., Jang Y.I., Chiang Y.M., Sadoway D.R., and Mayes A.M.: Rubbery block copolymer electrolytes for solid-state rechargeable lithium batteries. J. Electrochem. Soc. 146, 32 (1999).
Mitsuda H., Uno T., Kubo M., and Itoh T.: Solid polymer electrolytes based on poly(1,3-diacetyl-4-imidazolin-2-one). Polym. Bull. 57, 313 (2006).
Itoh T., Fujita K., Inoue K., Iwama H., Kondoh K., Uno T., and Kubo M.: Solid polymer electrolytes based on alternating copolymers of vinyl ethers with methoxy oligo(ethyleneoxy)ethyl groups and vinylene carbonate. Electrochim. Acta 112, 221 (2013).
Wang P., Chai J., Zhang Z., Zhang H., Ma Y., Xu G., Du H., Liu T., Li G., and Cui G.: An intricately designed poly(vinylene carbonate-acrylonitrile) copolymer electrolyte enables 5 V lithium batteries. J. Mater. Chem. A 7, 5295 (2019).
Britz J., Meyer W.H., and Wegner G.: Blends of poly(meth)acrylates with 2-oxo-(1,3)dioxolane side chains and lithium salts as lithium ion conductors. Macromolecules 40, 7558 (2007).
Tominaga Y.: Ion-conductive polymer electrolytes based on poly(ethylene carbonate) and its derivatives. Polym. J. 49, 291 (2017).
Spiegel E.F., Adamic K.J., Williams B.D., and Sammells A.F.: Solvation of lithium salts within single-phase dimethyl siloxane bisphenol—A carbonate block copolymer. Polymer 41, 3365 (2000).
Matsumoto M., Uno T., Kubo M., and Itoh T.: Polymer electrolytes based on polycarbonates and their electrochemical and thermal properties. Ionics 19, 615 (2013).
Abdul-Karim R., Hameed A., and Malik M.I.: Ring-opening polymerization of ethylene carbonate: Comprehensive structural elucidation by 1D & 2D-NMR techniques, and selectivity analysis. RSC Adv. 7, 11786 (2017).
Lee J.-C. and Litt M.H.: Ring-opening polymerization of ethylene carbonate and depolymerization of poly(ethylene oxide-co-ethylene carbonate). Macromolecules 33, 1618 (2000).
Dukhanin G.P., Dumler S.A., Sablin A.N., and Novakov I.A.: Solid polymeric electrolyte based on poly(ethylene carbonate)-lithium perchlorate system. Russ. J. Appl. Chem. 82, 243 (2009).
Tominaga Y., Nanthana V., and Tohyama D.: Ionic conduction in poly(ethylene carbonate)-based rubbery electrolytes including lithium salts. Polym. J. 44, 1155 (2012).
Kimura K., Hassoun J., Panero S., Scrosati B., and Tominaga Y.: Electrochemical properties of a poly(ethylene carbonate)-LiTFSI electrolyte containing a pyrrolidinium-based ionic liquid. Ionics 21, 895 (2015).
Kimura K., Matsumoto H., Hassoun J., Panero S., Scrosati B., and Tominaga Y.: A quaternary poly(ethylene carbonate)-lithium bis(trifluoromethanesulfonyl)imide-ionic liquid-silica fiber composite polymer electrolyte for lithium batteries. Electrochim. Acta 175, 134 (2015).
Motomatsu J., Kodama H., Furukawa T., and Tominaga Y.: Dielectric relaxation behavior of a poly(ethylene carbonate)-lithium bis-(trifluoromethanesulfonyl) imide electrolyte. Macromol. Chem. Phys. 216, 1660 (2015).
Tominaga Y., Yamazaki K., and Nanthana V.: Effect of anions on lithium ion conduction in poly(ethylene carbonate)-based polymer electrolytes. J. Electrochem. Soc. 162, A3133 (2015).
Kimura K., Motomatsu J., and Tominaga Y.: Highly concentrated polycarbonate-based solid polymer electrolytes having extraordinary electrochemical stability. J. Polym. Sci., Part B: Polym. Phys. 54, 2442 (2016).
Kimura K., Yajima M., and Tominaga Y.: A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem. Commun. 66, 46 (2016).
Morioka T., Ota K., and Tominaga Y.: Effect of oxyethylene side chains on ion-conductive properties of polycarbonate-based electrolytes. Polymer 84, 21 (2016).
Morioka T., Nakano K., and Tominaga Y.: Ion-conductive properties of a polymer electrolyte based on ethylene carbonate/ethylene oxide random copolymer. Macromol. Rapid Commun. 38, 1600652 (2017).
Motomatsu J., Kodama H., Furukawa T., and Tominaga Y.: Dielectric relaxation and ionic transport in poly(ethylene carbonate)-based electrolytes. Polym. Adv. Technol. 28, 362 (2017).
Kimura K. and Tominaga Y.: Understanding electrochemical stability and lithium ion-dominant transport in concentrated poly(ethylene carbonate) electrolyte. ChemElectroChem 5, 4008 (2018).
Munshi M.Z.A., Owens B.B., and Nguyen S.: Measurement of Li+ ion transport numbers in poly(ethylene oxide)–LiX complexes. Polym. J. 20, 597 (1988).
Tominaga Y., Shimomura T., and Nakamura M.: Alternating copolymers of carbon dioxide with glycidyl ethers for novel ion-conductive polymer electrolytes. Polymer 51, 4295 (2010).
Nakamura M. and Tominaga Y.: Utilization of carbon dioxide for polymer electrolytes [II]: Synthesis of alternating copolymers with glycidyl ethers as novel ion-conductive polymers. Electrochim. Acta 57, 36 (2011).
Smith M.J., Silva M.M., Cerqueira S., and MacCallum J.R.: Preparation and characterization of a lithium ion conducting electrolyte based on poly(trimethylene carbonate). Solid State Ionics 140, 345 (2001).
Manuela Silva M., Barbosa P., Evans A., and Smith M.J.: Novel solid polymer electrolytes based on poly(trimethylene carbonate) and lithium hexafluoroantimonate. Solid State Sci. 8, 1318 (2006).
Sun B., Mindemark J., Edström K., and Brandell D.: Realization of high performance polycarbonate-based Li polymer batteries. Electrochem. Commun. 52, 71 (2015).
Sun B., Xu C., Mindemark J., Gustafsson T., Edström K., and Brandell D.: At the polymer electrolyte interfaces: The role of the polymer host in interphase layer formation in Li-batteries. J. Mater. Chem. A 3, 13994 (2015).
Sun B., Mindemark J., Morozov E.V., Costa L.T., Bergman M., Johansson P., Fang Y., Furó I., and Brandell D.: Ion transport in polycarbonate based solid polymer electrolytes: Experimental and computational investigations. Phys. Chem. Chem. Phys. 18, 9504 (2016).
Meabe L., Lago N., Rubatat L., Li C., Müller A.J., Sardon H., Armand M., and Mecerreyes D.: Polycondensation as a versatile synthetic route to aliphatic polycarbonates for solid polymer electrolytes. Electrochim. Acta 237, 259 (2017).
He W., Cui Z., Liu X., Cui Y., Chai J., Zhou X., Liu Z., and Cui G.: Carbonate-linked poly(ethylene oxide) polymer electrolytes towards high performance solid state lithium batteries. Electrochim. Acta 225, 151 (2017).
Liu X., Ding G., Zhou X., Li S., He W., Chai J., Pang C., Liu Z., and Cui G.: An interpenetrating network poly(diethylene glycol carbonate)-based polymer electrolyte for solid state lithium batteries. J. Mater. Chem. A 5, 11124 (2017).
Jung Y.-C., Park M.-S., Kim D.-H., Ue M., Eftekhari A., and Kim D.-W.: Room-temperature performance of poly(ethylene ether carbonate)-based solid polymer electrolytes for all-solid-state lithium batteries. Sci. Rep. 7, 17482 (2017).
Melchiors M., Keul H., and Höcker H.: Preparation and properties of solid electrolytes on the basis of alkali metal salts and poly(2,2-dimethyltrimethylene carbonate)-block-poly(ethylene oxide)-block-poly(2,2-dimethyltrimethylene carbonate). Polymer 37, 1519 (1996).
Elmér A.M. and Jannasch P.: Synthesis and characterization of poly(ethylene oxide-co-ethylene carbonate) macromonomers and their use in the preparation of crosslinked polymer electrolytes. J. Polym. Sci., Part A: Polym. Chem. 44, 2195 (2006).
Yu X., Xiao M., Wang S., Han D., and Meng Y.: Fabrication and properties of crosslinked poly(propylene carbonate maleate) gel polymer electrolyte for lithium-ion battery. J. Appl. Polym. Sci. 118, 2078 (2010).
Kwon S.-J., Kim D.-G., Shim J., Lee J.H., Baik J.-H., and Lee J.-C.: Preparation of organic/inorganic hybrid semi-interpenetrating network polymer electrolytes based on poly(ethylene oxide-co-ethylene carbonate) for all-solid-state lithium batteries at elevated temperatures. Polymer 55, 2799 (2014).
Deng K., Wang S., Ren S., Han D., Xiao M., and Meng Y.: A novel single-ion-conducting polymer electrolyte derived from CO2-based multifunctional polycarbonate. ACS Appl. Mater. Interfaces 8, 33642 (2016).
Meabe L., Goujon N., Li C., Armand M., Forsyth M., and Mecerreyes D.: Single-ion conducting poly(ethylene oxide carbonate) as solid polymer electrolyte for lithium batteries. Batteries Supercaps 3, 68 (2020).
Huang X., Huang J., Wu J., Yu X., Gao Q., Luo Y., and Hu H.: Fabrication and properties of polybutadiene rubber-interpenetrating cross-linking poly(propylene carbonate) network as gel polymer electrolytes for lithium-ion battery. RSC Adv. 5, 52978 (2015).
Huang X., Zeng S., Liu J., He T., Sun L., Xu D., Yu X., Luo Y., Zhou W., and Wu J.: High-performance electrospun poly(vinylidene fluoride)/poly(propylene carbonate) gel polymer electrolyte for lithium-ion batteries. J. Phys. Chem. C 119, 27882 (2015).
Zhao J., Zhang J., Hu P., Ma J., Wang X., Yue L., Xu G., Qin B., Liu Z., Zhou X., and Cui G.: A sustainable and rigid-flexible coupling cellulose-supported poly(propylene carbonate) polymer electrolyte towards 5 V high voltage lithium batteries. Electrochim. Acta 188, 23 (2016).
Shin J.-H., Henderson W.A., and Passerini S.: Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes. Electrochem. Commun. 5, 1016 (2003).
Shin J.-H., Henderson W.A., and Passerini S.: PEO-based polymer electrolytes with ionic liquids and their use in lithium metal-polymer electrolyte batteries. J. Electrochem. Soc. 152, A978 (2005).
Wu H., Xu Y., Ren X., Liu B., Engelhard M.H., Ding M.S., El-Khoury P.Z., Zhang L., Li Q., Xu K., Wang C., Zhang J.-G., and Xu W.: Polymer-in-“Quasi-ionic liquid” electrolytes for high-voltage lithium metal batteries. Adv. Energy Mater. 9, 1902108 (2019).
Zhou D., Zhou R., Chen C., Yee W.-A., Kong J., Ding G., and Lu X.: Non-volatile polymer electrolyte based on poly(propylene carbonate), ionic liquid, and lithium perchlorate for electrochromic devices. J. Phys. Chem. B 117, 7783 (2013).
Zhang J., Zang X., Wen H., Dong T., Chai J., Li Y., Chen B., Zhao J., Dong S., Ma J., Yue L., Liu Z., Guo X., Cui G., and Chen L.: High-voltage and free-standing poly(propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery. J. Mater. Chem. A 5, 4940 (2017).
He Z., Chen L., Zhang B., Liu Y., and Fan L.: Flexible poly(ethylene carbonate)/garnet composite solid electrolyte reinforced by poly(vinylidene fluoride-hexafluoropropylene) for lithium metal batteries. J. Power Sources 392, 232 (2018).
Imholt L., Dörr T.S., Zhang P., Ibing L., Cekic-Laskovic I., Winter M., and Brunklaus G.: Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Sources 409, 148 (2019).
Zhu M., Wu J., Wang Y., Song M., Long L., Siyal S.H., Yang X., and Sui G.: Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 37, 126 (2019).
Florjańczyk Z., Zygadło-Monikowska E., Wieczorek W., Ryszawy A., Tomaszewska A., Fredman K., Golodnitsky D., Peled E., and Scrosati B.: Polymer-in-salt electrolytes based on acrylonitrile/butyl acrylate copolymers and lithium salts. J. Phys. Chem. B 108, 14907 (2004).
Łasińska A.K., Marzantowicz M., Dygas J.R., Krok F., Florjańczyk Z., Tomaszewska A., Zygadło-Monikowska E., Żukowska Z., and Lafont U.: Study of ageing effects in polymer-in-salt electrolytes based on poly(acrylonitrile-co-butyl acrylate) and lithium salts. Electrochim. Acta 169, 61 (2015).
Florjańczyk Z., Zygadło-Monikowska E., Affek A., Tomaszewska A., Łasińska A., Marzantowicz M., Dygas J.R., and Krok F.: Polymer electrolytes based on acrylonitrile–butyl acrylate copolymers and lithium bis(trifluoromethanesulfone)imide. Solid State Ionics 176, 2123 (2005).
Wu I.D. and Chang F.-C.: Determination of the interaction within polyester-based solid polymer electrolyte using FTIR spectroscopy. Polymer 48, 989 (2007).
Ravi M., Song S.-H., Gu K.-M., Tang J.-N., and Zhang Z.-Y.: Effect of lithium thiocyanate addition on the structural and electrical properties of biodegradable poly(ε-caprolactone) polymer films. Ionics 21, 2171 (2015).
Ravi M., Song S., Gu K., Tang J., and Zhang Z.: Electrical properties of biodegradable poly(ɛ-caprolactone): Lithium thiocyanate complexed polymer electrolyte films. Mater. Sci. Eng., B 195, 74 (2015).
Polo Fonseca C. and Neves S.: Electrochemical properties of a biodegradable polymer electrolyte applied to a rechargeable lithium battery. J. Power Sources 159, 712 (2006).
Fonseca C.P., Rosa D.S., Gaboardi F., and Neves S.: Development of a biodegradable polymer electrolyte for rechargeable batteries. J. Power Sources 155, 381 (2006).
Lin C.-K. and Wu I.D.: Investigating the effect of interaction behavior on the ionic conductivity of polyester/LiClO4 blend systems. Polymer 52, 4106 (2011).
Watanabe M., Togo M., Sanui K., Ogata N., Kobayashi T., and Ohtaki Z.: Ionic conductivity of polymer complexes formed by poly(β-propiolactone) and lithium perchlorate. Macromolecules 17, 2908 (1984).
Watanabe M., Rikukawa M., Sanui K., Ogata N., Kato H., Kobayashi T., and Ohtaki Z.: Ionic conductivity of polymer complexes formed by poly(ethylene succinate) and lithium perchlorate. Macromolecules 17, 2902 (1984).
Watanabe M., Rikukawa M., Sanui K., and Ogata N.: Effects of polymer structure and incorporated salt species on ionic conductivity of polymer complexes formed by aliphatic polyester and alkali metal thiocyanate. Macromolecules 19, 188 (1986).
Dupon R., Papke B.L., Ratner M.A., and Shriver D.F.: Ion transport in the polymer electrolytes formed between poly(ethylene succinate) and lithium tetrafluoroborate. J. Electrochem. Soc. 131, 586 (1984).
Lee Y.-C., Ratner M.A., and Shriver D.F.: Ionic conductivity in the poly(ethylene malonate)/lithium triflate system. Solid State Ionics 138, 273 (2001).
Pesko D.M., Jung Y., Hasan A.L., Webb M.A., Coates G.W., Miller T.F., and Balsara N.P.: Effect of monomer structure on ionic conductivity in a systematic set of polyester electrolytes. Solid State Ionics 289, 118 (2016).
Webb M.A., Jung Y., Pesko D.M., Savoie B.M., Yamamoto U., Coates G.W., Balsara N.P., Wang Z.-G., and Miller T.F.: Systematic computational and experimental investigation of lithium-ion transport mechanisms in polyester-based polymer electrolytes. ACS Cent. Sci. 1, 198 (2015).
Van Horn R.M., Steffen M.R., and O’Connor D.: Recent progress in block copolymer crystallization. Polym. Cryst. 1, e10039 (2018).
Mindemark J., Törmä E., Sun B., and Brandell D.: Copolymers of trimethylene carbonate and ε-caprolactone as electrolytes for lithium-ion batteries. Polymer 63, 91 (2015).
Acknowledgments
The authors gratefully acknowledge the financial support from National Key R&D Program of China (2018YFB0104300), Shenzhen Key Laboratory of Solid State Batteries (ZDSYS201802081843465), and Guangdong Provincial Key Laboratory of Energy Materials for Electric Power (2018B030322001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, H., Xie, J., Liu, Z. et al. Carbonyl-coordinating polymers for high-voltage solid-state lithium batteries: Solid polymer electrolytes. MRS Energy & Sustainability 7, 1 (2020). https://doi.org/10.1557/mre.2020.3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/mre.2020.3