Abstract
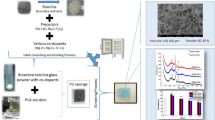
The development of synthetic scaffolds with a desirable combination of properties, such as bioactivity, the ability to locally deliver antibacterial agents and high osteogenic capacity, is a challenging but promising approach in bone tissue engineering. In this study, scaffolds of a borosilicate bioactive glass (composition: 6Na2O, 8K2O, 8MgO, 22CaO, 36B2O3, 18SiO2, 2P2O5; mol%) with controllable antibacterial activity were developed by doping the parent glass with varying amounts of Ag2O (0.05, 0.5, and 1.0 wt%). The addition of the Ag2O lowered the compressive strength and degradation of the bioactive glass scaffolds but it did not affect the formation of hydroxyapatite on the surface of the glass as determined by energy dispersive x-ray analysis, x-ray diffraction, and Fourier transform infrared analysis. The Ag2O-doped scaffolds showed a sustained release of Ag ions over more than 8 weeks in simulated body fluid and resistance against colonization by the bacterial strains Escherichia coli and Staphylococcus aureus. In vitro cell culture showed better adhesion, proliferation, and alkaline phosphatase activity of murine osteoblastic MC3T3-E1 cells on the Ag2O-doped bioactive glass scaffolds than on the undoped scaffolds. The results indicate that these Ag-doped borosilicate bioactive glass scaffolds may have potential in repairing bone coupled with providing a lower risk of bacterial infection.












Similar content being viewed by others
References
F. Yang, J. Wang, J. Hou, H. Guo, and C.S. Liu: Bone regeneration using cell-mediated responsive degradable PEG-based scaffolds incorporating with rhBMP-2. Biomaterials 34(5), 1514 (2013).
L. Pauksch, S. Hartmann, M. Rohnke, G. Szalay, V. Alt, R. Schnettler, and K.S. Lips: Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 10(1), 439 (2014).
A. Renaud, M. Lavigne, and P-A. Vendittoli: Periprosthetic joint infections at a teaching hospital in 1990–2007. Can. J. Surg. 55(6), 394 (2012).
A.M. Henslee, P.P. Spicer, D.M. Yoon, M.B. Nair, V.V. Meretoja, K.E. Witherel, J.A. Jansen, A.G. Mikos, and F.K. Kasper: Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 7(10), 3627 (2011).
J.H. Ye, Y.J. Xu, J. Gao, S.G. Yan, J. Zhao, Q.S. Tu, J. Zhang, X.J. Duan, C.A. Sommer, G. Mostoslavsky, D.L. Kaplan, Y.N. Wu, C.P. Zhang, L. Wang, and J. Chen: Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials 32(22), 5065 (2011).
W. Cao and L.L. Hench: Bioactive materials. Ceram. Int. 22(6), 493 (1996).
O. Tsigkou, J.R. Jones, J.M. Polak, and M.M. Stevens: Differentiation of fetal osteoblasts and formation of mineralized bone nodules by 45S5 Bioglass® conditioned medium in the absence of osteogenic supplements. Biomaterials 30(21), 3542 (2009).
J. Sun, L. Wei, X. Liu, J. Li, B. Li, G. Wang, and F. Meng: Influences of ionic dissolution products of dicalcium silicate coating on osteoblastic proliferation, differentiation and gene expression. Acta Biomater. 5(4), 1284 (2009).
W. Liang, M.N. Rahaman, D.E. Day, N.W. Marion, G.C. Riley, and J.J. Mao: Bioactive borate glass scaffold for bone tissue engineering. J. Non-Cryst. Solids 354(15), 1690 (2008).
X. Zhang, W. Jia, Y. Gu, W. Xiao, X. Liu, D. Wang, C. Zhang, W. Huang, M.N. Rahaman, and D.E. Day: Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 31(22), 5865 (2010).
X. Han and D.E. Day: Reaction of sodium calcium borate glasses to form hydroxyapatite. J. Mater. Sci.: Mater. Med. 18(9), 1837 (2007).
W. Huang, D.E. Day, K. Kittiratanapiboon, and M.N. Rahaman: Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci.: Mater. Med. 17(7), 583 (2006).
F.H. Nielsen: The emergence of boron as nutritionally important throughout the life cycle. Nutrition 16(7), 512 (2000).
L.A.H. Durand, A. Gongora, J.M.P. Lopez, A.R. Boccaccini, M.P. Zago, A. Baldi, and A. Gorustovich: In vitro endothelial cell response to ionic dissolution products from boron-doped bioactive glass in the SiO2-CaO-P2O5-Na2O system. J. Mater. Chem. B 2(43), 7620 (2014).
R. Forrer, C. Wenker, K. Gautschi, and H. Lutz: Concentration of 17 trace elements in serum and whole blood of plains viscachas (Lagostomus maximus) by ICP-MS, their reference ranges, and their relation to cataract. Biol. Trace Elem. Res. 81(1), 47 (2001).
R. Forrer, K. Gautschi, and H. Lutz: Simultaneous measurement of the trace elements Al, As, B, Be, Cd, Co, Cu, Fe, Li, Mn, Mo, Ni, Rb, Se, Sr, and Zn in human serum and their reference ranges by ICP-MS. Biol. Trace Elem. Res. 80(1), 77 (2001).
L. Bi, S. Jung, D. Day, K. Neidig, V. Dusevich, D. Eick, and L. Bonewald: Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J. Biomed. Mater. Res., Part A 100(12), 3267 (2012).
A.G. Gristina, P.T. Naylor, and Q.N. Myrvik: Biomaterial-centered infections: Microbial adhesion versus tissue integration. In Pathogenesis of Wound and Biomaterial-Associated Infections, (Springer, London, UK, 1990); p. 193.
O. Choi, K.K. Deng, N-J. Kim, L. Ross, Jr., R.Y. Surampalli, and Z. Hu: The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 42(12), 3066 (2008).
K. Kalishwaralal, S. BarathManiKanth, S.R.K. Pandian, V. Deepak, and S. Gurunathan: Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf., B 79(2), 340 (2010).
D.J.F. Moojen, S.N. Spijkers, C.S. Schot, M.W. Nijhof, H.C. Vogely, A. Fleer, A.J. Verbout, R.M. Castelein, W.J. Dhert, and L.M. Schouls: Identification of orthopaedic infections using broad-range polymerase chain reaction and reverse line blot hybridization. J. Bone Jt. Surg. 89(6), 1298 (2007).
L. Harris, S. Tosatti, M. Wieland, M. Textor, and R. Richards: Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly (l-lysine)-grafted-poly (ethylene glycol) copolymers. Biomaterials 25(18), 4135 (2004).
J.L. Clement and P.S. Jarrett: Antibacterial silver. Met.-Based Drugs 1(5–6), 467 (1994).
S. Percival, P. Bowler, and D. Russell: Bacterial resistance to silver in wound care. J. Hosp. Infect. 60(1), 1 (2005).
X. Liu, W. Huang, H. Fu, A. Yao, D. Wang, H. Pan, and W.W. Lu: Bioactive borosilicate glass scaffolds: Improvement on the strength of glass-based scaffolds for tissue engineering. J. Mater. Sci.: Mater. Med. 20(1), 365 (2009).
T. Kokubo and H. Takadama: How useful is SBF in predicting in vivo bone bioactivity?Biomaterials 27(15), 2907 (2006).
G.N. King, N. King, and F.J. Hughes: Effect of two delivery systems for recombinant human bone morphogenetic protein-2 on periodontal regeneration in vivo. J. Periodontal Res. 33(3), 226 (1998).
F. Zheng, S. Wang, S. Wen, M. Shen, M. Zhu, and X. Shi: Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly (lactic-co-glycolic acid) composite nanofibers. Biomaterials 34(4), 1402 (2013).
M. Erol, V. Mouriňo, P. Newby, X. Chatzistavrou, J. Roether, L. Hupa, and A.R. Boccaccini: Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 8(2), 792 (2012).
A. Boronin, S. Koscheev, and G. Zhidomirov: XPS and UPS study of oxygen states on silver. J. Electron Spectrosc. Relat. Phenom. 96(1–3), 43 (1998).
X. Liu, M.N. Rahaman, and D.E. Day: Conversion of melt-derived microfibrous borate (13-93B3) and silicate (45S5) bioactive glass in a simulated body fluid. J. Mater. Sci.: Mater. Med. 24(3), 583 (2013).
J.R. Jones and L.L. Hench: Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J. Biomed. Mater. Res., Part B 68(1), 36 (2004).
Z. Ma, M. Kotaki, R. Inai, and S. Ramakrishna: Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 11(1–2), 101 (2005).
C. Baer, R. Foldbjerg, Y. Hayashi, D.S. Sutherlans, and H. Autrup: Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 208, 286 (2012).
S. Kittler, C. Greulich, J. Diendorf, M. Koller, and M. Epple: Toxicity of silver nanoparticles increases during storage because of dissolution of slow dissolution under release of silver ions. Chem. Mater. 22, 4548 (2010).
M.V. Park, A.M. Neigh, J.P. Vermeulen, L.J. de la Fonteyne, H.W. Verharen, J.J. Briedé, and H. van Loveren and W.H. de Jong: The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32(36), 9810 (2011).
P. AshaRani, G. Low Kah Mun, M.P. Hande, and S. Valiyaveettil: Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2), 279 (2008).
M. Rai, A. Yadav, and A. Gade: Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27(1), 76 (2009).
C.E. Albers, W. Hofstetter, K.A. Siebenrock, R. Landmann, and F.M. Klenke: In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 7(1), 30 (2013).
A. Ewald, D. Hösel, S. Patel, L.M. Grover, J.E. Barralet, and U. Gbureck: Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 7(11), 4064 (2011).
C.D. Hunt: Dietary boron: Progress in establishing essential roles in human physiology. J. Trace Elem. Med. Biol. 26(2), 157 (2012).
M. Park, Q. Li, N. Shcheynikov, W. Zeng, and S. Muallem: NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol. Cell 16(3), 331 (2004).
A.A. Gorustovich, J.M.P. López, M.B. Guglielmotti, and R.L. Cabrini: Biological performance of boron-modified bioactive glass particles implanted in rat tibia bone marrow. Biomed. Mater. 1(3), 100 (2006).
K.M. Reddy, K. Feris, J. Bell, D.G. Wingett, C. Hanley, and A. Punnoose: Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 90(21), 213902 (2007).
M.A. Kohanski, D.J. Dwyer, and J.J. Collins: How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 8(6), 423 (2010).
K.A. Brogden: Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria?Nat. Rev. Microbiol. 3(3), 238 (2005).
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Commission of Shanghai Municipality (Grant No. 12JC1408500) and the National Natural Science Foundation, China (Grant Nos. 51072133, 81201377 and 51372170).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, H., Zhao, S., Cui, X. et al. Evaluation of three-dimensional silver-doped borate bioactive glass scaffolds for bone repair: Biodegradability, biocompatibility, and antibacterial activity. Journal of Materials Research 30, 2722–2735 (2015). https://doi.org/10.1557/jmr.2015.243
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.243