Abstract
Background
The Oncotype DX® assay has been validated in predicting response to adjuvant chemotherapy in breast cancer. Its role in neoadjuvant chemotherapy (NCT) has not been established.
Methods
The National Cancer Database was used to identify all patients with T1–T3, ER-positive, HER2-negative primary invasive breast cancer diagnosed from 2010 to 2015 who had Oncotype DX recurrence scores (RS) and received NCT. RS were classified as low, intermediate, or high. Unadjusted and adjusted regression analyses were performed to determine the association between pathologic complete response (pCR) and RS.
Results
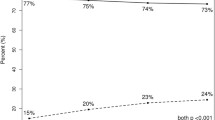
A total of 989 patients (mean age, 54.6 years) with available RS who underwent NCT were identified. RS were low in 227 (23.0%) patients, intermediate in 450 (45.5%) patients, and high in 312 (31.5%) patients. Most patients had a T1 (431 [43.6%]) or T2 tumor (451 [45.6%]). Most had N0 disease (757 [76.5%]). Tumor grades were 1 (123 [12.4%]), 2 (517 [52.3%]), or 3 (349 [35.3%]). pCR was achieved by 42 (4.3%) patients. Adjusted multivariable analysis showed a significant association between pCR and high RS (odds ratio 4.87; 95% confidence interval 2.01–11.82).
Conclusions
High Oncotype DX RS was associated with pCR after NCT in this national cohort of ER-positive, HER2-negative patients. Oncotype DX testing could help to identify patients most suited for NCT and should be considered for incorporation into the multidisciplinary decision-making process.

Similar content being viewed by others
References
Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol 2017;52:56–73.
Győrffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res BCR 2015;17(1):11.
Genomic Health, Inc. Announces Publication of Comprehensive Economic Analysis Confirming Cost-Effectiveness of Oncotype DX™ in the American Journal of Managed Care [press release]. Redwood City, CA, May 13, 2005.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817–26.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor–positive breast cancer. J Clin Oncol 2006;24(23):3726–34.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28(11):1829–34.
Riba LA, Gruner RA, Fleishman A, James TA. Surgical risk factors for the delayed initiation of adjuvant chemotherapy in breast cancer. Ann Surg Oncol 2018;25:1904–11.
Kantor O, Sipsy LM, Yao K, James TA. A Predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018;25:1304–11.
Ajmani GS, James TA, Kantor O, Wang CH, Yao KA. The impact of facility volume on rates of pathologic complete response to neoadjuvant chemotherapy used in breast cancer. Ann Surg Oncol 2017;24(11):3157–66.
Haddad TC, Goetz MP. Landscape of neoadjuvant therapy for breast cancer. Ann Surg Oncol 2015;22(5):1408–15.
Anderson J, Shak S, Millward C, et al. Molecular characterization of breast cancer core biopsy specimens by gene expression analysis using standardized quantitative RT-PCR. Cancer Res 2009;69(24 Suppl):6021–1.
Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 2005;23(29):7265–77.
Chang JC, Makris A, Gutierrez MC, et al. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Cancer Res Treat 2008;108(2):233–40.
American College of Surgeons. National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb. Accessed 20 Dec 2017.
Yardley DA, Peacock NW, Shastry M, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res Treat 2015;154(2):299–308.
Mina L, Soule SE, Badve S, et al. Predicting response to primary chemotherapy: gene expression profiling of paraffin-embedded core biopsy tissue. Breast Cancer Res Treat 2007;103(2):197–208.
Soran A, Bhargava R, Johnson R, et al. The impact of Oncotype DX(R) recurrence score of paraffin-embedded core biopsy tissues in predicting response to neoadjuvant chemotherapy in women with breast cancer. Breast Dis 2016;36(2–3):65–71.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 2014;384(9938):164–72.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11(1):55–65.
Brufsky AM. Predictive and prognostic value of the 21-gene recurrence score in hormone receptor–positive, node-positive breast cancer. Am J Clin Oncol 2014;37(4):404–10.
Jasem J, Fisher CM, Amini A, et al. The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER Trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Cancer Netw JNCCN 2017;15(4):494–503.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305(6):569–75.
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15(12):1303–10.
Riba LA, Gruner RA, Fleishman A, James TA. Surgical risk factors for the delayed initiation of adjuvant chemotherapy in breast cancer. Ann Surg Oncol 2018;25(7):1904–11.
US Food and Drug Administration. Guidance for Industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. October 2014.
Acknowledgement
The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Pease, A.M., Riba, L.A., Gruner, R.A. et al. Oncotype DX® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann Surg Oncol 26, 366–371 (2019). https://doi.org/10.1245/s10434-018-07107-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07107-8