Abstract
Background
Positive surgical margins remain a significant challenge in breast cancer surgery. This report describes the use of a novel, first-in-human ratiometric activatable cell-penetrating peptide in breast cancer surgery.
Methods
A two-part, multi-institutional phase 1 trial of AVB-620 with a 3+3 dose escalation and dose-expansion cohorts was conducted. The patients received an infusion of AVB-620 2–20 h before planned lumpectomy/mastectomy and sentinel node biopsy/axillary dissection. Imaging analysis was performed on images obtained from the surgical field as well as post-excision surgical specimens. Pathology reports were obtained to correlate imaging results with histopathologic data. Information on physical adverse events and laboratory abnormalities were recorded.
Results
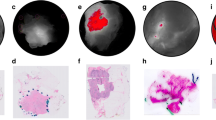
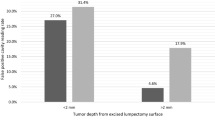
A total of 27 patients received infusion of AVB-620 and underwent surgical excision of breast cancer. The findings showed no adverse events or laboratory values attributable to infusion of AVB-620. The 8-mg dose was selected from the dose-escalation cohort for use with the expansion cohort based on imaging data. Region-of-interest (ROI) imaging analysis from the 8-mg cohort demonstrated measurable changes between pathology confirmed tumor-positive and tumor-negative tissue.
Conclusion
Intraoperative imaging of surgical specimens after infusion with AVB-620 allowed for real-time tumor detection. Infusion of AVB-620 is safe and may improve intraoperative detection of malignant tissue during breast cancer operations.




Similar content being viewed by others
Change history
31 July 2017
An erratum to this article has been published.
References
Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol. 2011;18:3407–14.
Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity-shave margins in breast cancer. N Engl J Med. 2015;373:503–10.
Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014;156:190–97.
Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol. 2012;19:3236–45.
Schulman AM, Mirrielees JA, Leverson G, Landercasper J, Greenberg C, Wilke LG. Reexcision surgery for breast cancer: an analysis of the American Society of Breast Surgeons (ASBrS) MasterySM Database following the SSO-ASTRO “no-ink-on-tumor” guidelines. Ann Surg Oncol. 2017;24:52–8.
Zysk AM, Chen K, Gabrielson E, et al. Intraoperative assessment of final margins with a handheld optical imaging probe during breast-conserving surgery may reduce the reoperation rate: results of a multicenter study. Ann Surg Oncol. 2015;22:3356–62.
Whitley MJ, Cardona DM, Lazarides AL, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med. 2016;8:320ra4. doi:10.1126/scitranslmed.aad0293.
Kennedy GT, Okusanya OT, Keating JJ, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg. 2015;262:602–09.
Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA 2004;101:17867–72.
Savariar EN, Felsen CN, Nashi N, et al. Real-time in vivo molecular detection of primary tumors and metastases with ratiometric activatable cell-penetrating peptides. Cancer Res. 2013;73:855–64.
Miampamba M, Liu J, Harootunian A, et al. Sensitive in vivo visualization of breast cancer using ratiometric protease-activatable fluorescent imaging agent, AVB-620. Theranostics (in press). doi:10.7150/thno.20678.
Gonzalez LO, Corte MD, Vazquez J, et al. Study of matrix metalloproteinases and their tissue inhibitors in ductal in situ carcinomas of the breast. Histopathology. 2008;53:403–15.
Olson ES, Aguilera TA, Jiang T, et al. In vivo characterization of activatable cell-penetrating peptides for targeting protease activity in cancer. Integr Biol. 2009;1:382–93.
Metildi CA, Felsen CN, Savariar EN, et al. Ratiometric activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models. Ann Surg Oncol. 2015;22:2082–87.
Laws A, Brar MS, Bouchard-Fortier A, Leong B, Quan ML. Intraoperative margin assessment in wire-localized breast-conserving surgery for invasive cancer: a population-level comparison of techniques. Ann Surg Oncol. 2016;23:3290–96.
Acknowledgment
The authors acknowledge the Clinical Trials Office at Moores Cancer Center for their administrative oversight. Avelas Biosciences, Inc. paid for the clinical trial and administrative support.
Disclosure
Steven L. Chen, Jesús E. González, and Alec Harootunian are employees of Avelas Biosciences, Inc and have financial stock options. Jonathan T. Unkart, Irene L. Wapnir, and Anne M. Wallace have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1245/s10434-017-6028-7.
Rights and permissions
About this article
Cite this article
Unkart, J.T., Chen, S.L., Wapnir, I.L. et al. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann Surg Oncol 24, 3167–3173 (2017). https://doi.org/10.1245/s10434-017-5991-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5991-3