ABSTRACT
Purpose
Melanoma patients with in-transit disease have a high mortality rate despite various treatment strategies. The aim of this study was to validate the role of intralesional interleukin (IL)-2, to understand its mechanism of action, and to better understand factors that may influence its response.
Methods
We retrospectively collected the clinicopathological data of 31 consecutive patients who presented to a tertiary care cancer center for treatment of in-transit melanoma with intralesional IL-2. Kaplan–Meier survival curves and multivariable Cox regression analysis were performed. Immunohistochemistry (IHC) was used to better understand the immune response to localized IL-2 therapy. Targeted next-generation sequencing was performed to genomically characterize the tumors.
Results
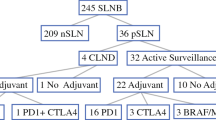
Ten patients (10/31, 32 %) achieved a pathologic complete response (pCR), 17/21 (55 %) had a partial response, and 4/21 (19 %) had progressive disease on treatment. pCR to IL-2 therapy was associated with overall survival (log-rank p = 0.004) and improved progression-free survival (PFS) [adjusted hazard ratio (HR) 0.11; 95 % CI 0.02–0.47; p = 0.003). A higher CD8+ T cell infiltrate was identified in in-transit lesions with a pCR compared with the other lesions (mean IHC score 3.78 vs. 2.61; p = 0.01). Patients with an elevated CD8+ infiltrate demonstrated an improved PFS (unadjusted HR 0.08; 95 % CI 0.01–0.52; p = 0.008).
Conclusions
Thirty-two percent of patients achieved pCR with intralesional IL-2 therapy and had a significantly improved PFS compared with the rest of the cohort, which may be explained by a systemic CD8+ T-cell response.


Similar content being viewed by others
References
Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2011. Bethesda: National Cancer Institute. Based on November 2013 SEER data submission, posted to the SEER website, April 2014.
Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6(3):315–21.
Meier F, Will S, Ellwanger U, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147(1):62–70.
Melanoma of the Skin. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer; 2010:325.
Speicher PJ, Tyler DS, Mosca PJ. Management of in-transit malignant melanoma. In: Duc GHT, ed. Melanoma: from early detection to treatment: InTech; 2013.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206.
Weide B, Faller C, Buttner P, et al. Prognostic factors of melanoma patients with satellite or in-transit metastasis at the time of stage III diagnosis. PLoS One. 2013;8(4):e63137.
Pawlik TM, Ross MI, Thompson JF, Eggermont AM, Gershenwald JE. The risk of in-transit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23(21):4588–90.
Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg. 2004;91(6):673–82.
Storm FK, Morton DL. Value of therapeutic hyperthermic limb perfusion in advanced recurrent melanoma of the lower extremity. Am J Surg. 1985;150(1):32–5.
Kroon BB, Klaase JM, van Geel BN, Eggermont AM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur J Cancer. 1993;29A(3):325–8.
Turley RS, Raymond AK, Tyler DS. Regional treatment strategies for in-transit melanoma metastasis. Surg Oncol Clin N Am. 2011;20(1):79–103.
Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 2010;15(4):416–27.
Mian R, Henderson MA, Speakman D, Finkelde D, Ainslie J, McKenzie A. Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can J Surg. 2001;44(3):189–92.
Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol. 2004;30(10):1107–12.
Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208(5):706–15; discussion 15-7.
Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15(8):2195–205.
Testori A, Faries MB, Thompson JF, et al. Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol. 2011;104(4):391–6.
Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180(4):635–43.
Kidner TB, Morton DL, Lee DJ, et al. Combined intralesional Bacille Calmette–Guerin (BCG) and topical imiquimod for in-transit melanoma. J Immunother. 2012;35(9):716–20.
Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156(2):337–45.
Utikal J, Zimpfer A, Thoelke A, et al. Complete remission of multiple satellite and in-transit melanoma metastases after sequential treatment with isolated limb perfusion and topical imiquimod. Br J Dermatol. 2006;155(2):488–91.
Otsu U, Fukui N, Iki M, Moriwaki S, Kiyokane K. Case of cutaneous malignant melanoma surviving 16 years with late recurrence. J Dermatol. 2009;36(11):598–603.
Boyd KU, Wehrli BM, Temple CL. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J Surg Oncol. 2011;104(7):711–7.
Ridolfi L, Ridolfi R, Ascari-Raccagni A, et al. Intralesional granulocyte–monocyte colony-stimulating factor followed by subcutaneous interleukin-2 in metastatic melanoma: a pilot study in elderly patients. J Eur Acad Dermatol Venereol. 2001;15(3):218–23.
Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–6.
Dehesa LA, Vilar-Alejo J, Valeron-Almazan P, Carretero G. Experience in the treatment of cutaneous in-transit melanoma metastases and satellitosis with intralesional interleukin-2 [in Spanish]. Actas Dermosifiliogr. 2009;100(7):571–85.
Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–46.
Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651–6.
Khalili JS, Liu S, Rodriguez-Cruz TG, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18(19):5329–40.
Byers BA, Temple-Oberle CF, Hurdle V, McKinnon JG. Treatment of in-transit melanoma with intra-lesional interleukin-2: a systematic review. J Surg Oncol. 2014;110(6):770–5.
Gutwald J, Groth W, Mahrle G. Peritumoral administered IL-2-induced tumor regression in melanoma. Pilot study [in German]. Hautarzt. 1994;45(8):536–40.
Temple-Oberle CF, Byers BA, Hurdle V, Fyfe A, McKinnon JG. Intra-lesional interleukin-2 therapy for in transit melanoma. J Surg Oncol. 2014;109(4):327–31.
Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29(12):1539–46.
Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol. 2012;30(20):2522–9.
Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–8.
Augustine CK, Jung SH, Sohn I, et al. Gene expression signatures as a guide to treatment strategies for in-transit metastatic melanoma. Mol Cancer Ther. 2010;9(4):779–90.
Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother. 2012;35(1):66–72.
Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–31.
Knight DA, Ngiow SF, Li M, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123(3):1371–81.
Donia M, Fagone P, Nicoletti F, et al. BRAF inhibition improves tumor recognition by the immune system: Potential implications for combinatorial therapies against melanoma involving adoptive T-cell transfer. Oncoimmunology. 2012;1(9):1476–83.
Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–31.
Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23 Suppl 8:viii41-6.
Nespoli L, Uggeri F, Romano F, et al. Modulation of systemic and intestinal immune response by interleukin-2 therapy in gastrointestinal surgical oncology. Personal experience in the context of current knowledge and future perspectives. Anticancer Res. 2012;32(3):989–96.
Den Otter W, Jacobs JJ, Battermann JJ, et al. Local therapy of cancer with free IL-2. Cancer Immunol Immunother. 2008;57(7):931–50.
Shaker MA, Younes HM. Interleukin-2: evaluation of routes of administration and current delivery systems in cancer therapy. J Pharm Sci. 2009;98(7):2268–98.
Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109(4):314–9.
Hersey P, Gallagher S. Intralesional immunotherapy for melanoma. J Surg Oncol. 2014;109(4):320–6.
Acknowledgment
The authors would like to thank the institutions across Ontario who helped provide paraffin-embedded blocks for this study, including University Health Network, Barrie Royal Victoria Hospital, Muskoka Algonquin Healthcare, Headwaters Health, Orillia Soldier’s Memorial Hospital, West Parry Sound Health Centre, Lifelabs, Gamma Dynacare, Windsor Regional Hospital, Ross Memorial Hospital, Peterborough Regional Health Centre, London Health Sciences Centre, St. Mary’s General Hospital, Kitchener, Kingston General Hospital, and Grand River Hospital. This study was supported by the William S. Fenwick Fellowship and the Joseph M. West Family Memorial Fund from the 2012 Postgraduate Medical Research Award, awarded to Saima Hassan, University of Toronto; Melanoma Site Group from Sunnybrook Health Sciences Centre, awarded to Teresa Petrella; and a Research Grant from Pathology Associates from the University Health Network, awarded to Danny Ghazarian.
Disclosure
Novartis provided interleukin-2 to patients free of charge as part of a compassionate release program. Novartis was not involved in funding this research study. Teresa Petrella and an immediate family member received honoraria from Novartis, Roche, BMS, Merck, GSK, Amgen, AstraZeneca, Electa, Janssen, Paladin, and Sanofi; they served as a consultant or in an advisory role for Novartis, Roche, BMS, Merck, GSK, Amgen, Astellas, Janssen, and Sanofi. An immediate family member of Teresa Petrella was paid to speak at a speaker’s bureau from Amgen, and has received research funding from Sanofi and Paladin. The University Health Network, Toronto, ON, Canada, where Suzanne Kamel-Reid is an employee, receives research funding from Novartis. Saima Hassan, Tong Zhang, Francesco Nordio, Andrea Baccarelli, Shachar Sade, Karen Naert, Ayman Al Habeeb, Danny Ghazarian, and Frances Wright have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, S., Petrella, T.M., Zhang, T. et al. Pathologic Complete Response to Intralesional Interleukin-2 Therapy Associated with Improved Survival in Melanoma Patients with In-Transit Disease. Ann Surg Oncol 22, 1950–1958 (2015). https://doi.org/10.1245/s10434-014-4199-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4199-z