Abstract
Purpose
To assess the impact of stroke volume index (SVI) at the end of esophagectomy upon postoperative renal function.
Methods
We reviewed medical records of 128 patients undergoing esophagectomy. Intraoperative hemodynamics were monitored with the FloTrac sensor/Vigileo monitor system in addition to standard monitors. Patients were divided into two groups according to SVI at the end of surgery: the normal SVI group (n = 76), with SVI ≥ 35 ml/m2, and the low SVI group (n = 52), with SVI < 35 ml/m2. We compared postoperative renal function, indicated by serum creatinine and estimated glomerular filtration rate, on postoperative days 0 through 3. We also compared numbers of patients who developed postoperative acute kidney injury (AKI).
Results
Although there were no intergroup differences in preoperative renal function or other intraoperative hemodynamic variables, including arterial pressure, central venous pressure, stroke volume variation, a volume of infusion, urine output, and the total intraoperative in–out balance, estimated glomerular filtration rate was significantly lower and serum creatinine was significantly higher in the low SVI group than in the normal SVI group on postoperative days 1 and 2 (P < 0.05). In addition, more patients developed postoperative AKI in the low SVI group than in the normal SVI group (12 of 52 vs. 5 of 76, P = 0.015).
Conclusions
Low SVI at the end of esophagectomy may represent a risk factor for AKI in the early postoperative period. Further studies are required to examine whether maintaining SVI above 35 ml/m2 reduces the incidence of AKI after esophagectomy.
Similar content being viewed by others
Postoperative renal dysfunction is a severe complication which is associated with poor outcome after surgery.1–3 The Acute Kidney Injury Network (AKIN) proposed the term acute kidney injury (AKI) to reflect the entire spectrum of acute renal failure.4 AKIN proposed refinements of the former criteria to increase sensitivity, recommending that a smaller increase in serum creatinine (Cr) should be sufficient to define the development of AKI (Table 1).4 The major causes of AKI include renal hypoperfusion, sepsis/systemic inflammatory response syndrome (SIRS), and direct nephrotoxicity, though in most cases, etiology is multifactorial.5–7 Providing adequate hydration in such situations is the key therapy for the prevention and treatment of AKI.6
The incidence of postoperative AKI varies according to the type of surgery.2 Radical esophagectomy for esophageal cancer is one of the most invasive noncardiac surgical procedures, which potentially induces SIRS characterized by the overproduction of proinflammatory cytokines.8,9 Fluid management is often difficult to gauge during esophagectomy, since depletion of intravascular volume is common due to a dynamic and extensive shift of extracellular fluid from central to peripheral compartments.8 On the other hand, deliberate restriction of fluid resuscitation is required during esophagectomy to reduce the risk of postoperative respiratory complications.10,11 AKI thus remains as one of the most common complications following esophagectomy.12,13
Mean arterial blood pressure (MAP), heart rate (HR), central venous pressure (CVP), urine output, and in–out balance have been used as indicators for intraoperative fluid management. However, in our experience, a significant number of patients following esophagectomy experience postoperative AKI despite a stable hemodynamic status during surgery. Therefore, fluid management using conventional hemodynamic indicators might not be sufficient to prevent AKI. Recently, the FloTrac sensor/Vigileo monitor system (Edwards Lifesciences, Irvine, CA, USA), a device that can determine cardiac index (CI), stroke volume index (SVI), and stroke volume variation (SVV) by arterial pulse contour analysis, was introduced into clinical practice.14 SVV is calculated from the change of stroke volume during mechanical ventilation.8,15 A decrease in SVV, and conversely an increase in SVI, in response to volume loading are useful indicators of the effectiveness of fluid management.8,15,16 SVI and SVV can thus be used as reliable indicators of fluid responsiveness or cardiac preloading.17 Such hemodynamic variables may be helpful in predicting and/or preventing AKI after esophagectomy. To date, however, no study has demonstrated significant association between SVI and SVV and the development of AKI after esophagectomy.
We started to use the FloTrac/Vigileo system routinely during esophagectomy in 2007. We conducted the current retrospective study in an attempt to identify a risk factor for postoperative AKI in the first 128 patients undergoing esophagectomy in whom this system was used intraoperatively. We found that SVI at the end of surgery closely correlated with the development of postoperative AKI. We therefore divided patients into two groups according to whether SVI at the end of surgery was normal or subnormal, then compared postoperative renal function between the two groups.
Materials and Methods
This retrospective study was approved by the institutional review board of Juntendo University Hospital. The review board specifically considered this retrospective chart review, including subject selection and confidentiality, and waived the need for patient consent.
Subjects
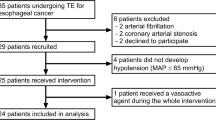
We reviewed the medical records of 139 adult patients who underwent radical esophagectomy involving cervico-thoraco-abdominal three-field lymph nodes dissection for esophageal cancer between 2007 and 2011 in our institution.
Exclusion criteria included cardiac diseases classified as New York Heart Association classes II to IV; respiratory dysfunction indicated by a vital capacity (VC) of <50 % or a forced expiratory volume in 1 s as percentage of forced VC (%FVC1.0) of <50 %; anemia (hemoglobin < 10 g/dl); thrombocytopenia (platelet count < 100,000/μl); coagulopathy (prothrombin time-international normalized ratio > 1.2); preexisting renal dysfunction indicated by an estimated glomerular filtration rate (eGFR) < 60 ml/min and/or Cr > 2.0 mg/dl; and late extubation in the intensive care unit (ICU).
Eleven patients were excluded according to the criteria, and the remaining 128 patients were included in the study.
Anesthesia and Surgery
No patients received premedication. All patients underwent combined general/epidural anesthesia. Electrocardiogram, MAP, CVP, pulse oximetry (SpO2), end-tidal CO2 tension, rectal temperature, and urine output were monitored during anesthesia. Arterial blood gas analyses were performed intermittently.
After placement of an epidural catheter via a lower thoracic intervertebral space, general anesthesia was induced with propofol (1–2 mg/kg) and remifentanil (0.3–0.5 μg/kg/min). Orotracheal intubation by a single-lumen reinforced tube (Mallinckrodt Oral/Nasal Tracheal Tube Cuffed Reinforced; Covidien, Mansfield, MA, USA) was facilitated with rocuronium (0.6–0.9 mg/kg) or vecuronium (0.1 mg/kg). Anesthesia was maintained with sevoflurane (1–2 % in end-tidal concentration), remifentanil (0.1–0.5 μg/kg/min), and epidural analgesia with 0.375 % ropivacaine and morphine (4–6 ml and 3 mg, respectively, as initial bolus doses). Lungs were ventilated with pressure control ventilation employing 5 cm H2O positive end-expiratory pressure (PEEP). Peak inspiratory pressure was maintained below 20 cm H2O and 30 cm H2O during bilateral lung ventilation and one-lung ventilation, respectively.
After induction of anesthesia, patients were placed in the left lateral position. During thoracic esophagectomy, one-lung ventilation was achieved with an endobronchial blocker tube (Endobronchial Blocker Tube; Coopdech, Osaka, Japan). After resection of the thoracic esophagus, bilateral lung ventilation was resumed, and the thoracic incision was closed. Patients were then placed in the supine position, and a gastric conduit was constructed. After closure of the abdominal wound, cervical esophagogastrostomy was completed.
An epidural infusion of 0.2 % ropivacaine (3 ml/h) and morphine (5 mg/day) was initiated before the end of general anesthesia and continued for 72 postoperative hours using a disposable pump (multirate infusor flow rate: 2, 3, 5 ml/h; Baxter, Deerfield, IL, USA). After surgery, all patients were extubated in the operating room and transferred to the ICU.
Crystalloids were infused to maintain stable intraoperative hemodynamics, primarily relying on conventional hemodynamic variables such as MAP, HR, and CVP, and to keep the total intraoperative in–out balance within 7–9 ml/kg/h. Red cell concentrates were transfused in accordance with clinical needs to maintain hemoglobin >7 g/dl. Postoperatively, crystalloids were infused at rates of 1.8–2.0, 1.6–1.8, and 1.3–1.5 ml/kg/h on postoperative day (POD) 0, POD1, and POD2–3, respectively, depending on the relatively strict perioperative fluid management protocol of the surgical team.
Sampling of Hemodynamic Data
After the induction of general anesthesia, invasive arterial blood pressure monitoring was initiated via a 22-gauge elastic catheter placed in the left radial artery and connected to the FloTrac/Vigileo system to measure CI, SVI, and SVV. These data were reported every 20 s and recorded via an off-line computer. MAP, HR, and CVP were recorded every minute within the anesthesia record system (Orsys; Philips International, Amsterdam, The Netherlands). By reviewing the records of the 128 subjects, MAP, HR, CVP, CI, SVI, and SVV at the end of surgery were determined as the means of variables recorded over the last 5 min of the surgery.
Sampling of Data on Renal Function
Renal function was evaluated with eGFR, which was calculated by use of the modification of diet in renal disease formula, in addition to Cr. Renal function was evaluated preoperatively and on POD0–3.18
Effects of SVI on Postoperative Renal Function
We examined correlation between hemodynamic data at the end of surgery and data pertaining to postoperative renal function. Because there was a significant correlation between SVI values at the end of surgery and postoperative eGFR or Cr, we divided patients into two groups according to whether SVI at the end of surgery was normal (SVI ≥ 35 ml/m2, normal SVI group, n = 76) or subnormal (SVI < 35 ml/m2, low SVI group, n = 52), on the basis of previous studies referring to the lower normal limit of SVI (35 ml/m2).16,19–21 Then we compared postoperative renal function and the incidence of postoperative AKI between the two groups.
Statistical Analysis
Data are expressed as mean ± standard deviation or numbers of patients. Statistical analyses were performed with the Kolmogorov–Smirnov test for examining data distribution, paired t test, one-way analysis of variance followed by Tukey–Kramer test, linear regression analysis, and Fisher’s exact test, as appropriate. Statistical differences were considered significant if P values were less than 0.05. Analyses were carried out with GraphPad Prism 5 statistical software (GraphPad Software Inc., San Diego, CA, USA).
Results
Seventy-six and 52 patients were classified into the normal and low SVI groups, respectively. There was no significant difference in demographic, anesthetic, or surgical data between the two groups (Table 2). Although SVI and CI at the end of surgery were significantly lower in the low SVI group than in the normal SVI group, other hemodynamic variables, including MAP, HR, CVP, and SVV, did not differ between the groups (Table 2).
Preoperatively, there was no significant difference in Cr or eGFR between the groups (Fig. 1). Although Cr was significantly increased and eGFR significantly reduced on POD1 and POD2 in both groups compared with preoperative values, Cr was significantly lower and eGFR was significantly higher in the normal SVI group than in the low SVI group on POD1 and POD2 (Fig. 1). Moreover, the incidence of postoperative AKI was higher in the low SVI group than in the normal SVI group (P = 0.015, Table 3).
Perioperative changes in eGFR (left) and Cr (right). Data are presented as mean ± standard deviation. Blue and yellow circles indicate the low and normal SVI groups, respectively. PreOp and POD0–3 indicate a preoperative day and postoperative days 0–3, respectively. *Lower than the preoperative value (P < 0.05). #Lower than the normal SVI group (P < 0.05). †Higher than the preoperative value (P < 0.05). $Higher than the normal SVI group (P < 0.05)
CI at the end of surgery positively correlated with eGFR on POD1 and POD2 (r = 0.41, P < 0.0001, and r = 0.39, P < 0.0001, respectively), and negatively correlated with Cr on POD1 and POD2 (r = −0.31, P < 0.001, and r = −0.30, P < 0.001, respectively). SVI at the end of surgery more closely correlated with eGFR on POD1 and POD2 (r = 0.51, P < 0.0001, and r = 0.46, P < 0.0001, respectively) (Fig. 2), and with Cr on POD1 and POD2 (r = −0.45, P < 0.0001, and r = −0.40, P < 0.0001, respectively) (Fig. 2). Other hemodynamic variables, including MAP, HR, CVP, and SVV, were not correlated with any variables relating to postoperative renal function.
Correlations between SVI and eGFR (left), and between SVI and Cr (right) on postoperative day 1 (POD1) (top) and POD2 (bottom). There were significant correlations between SVI and eGFR, and between SVI and Cr on POD1 and POD2. Correlation coefficient (r) and probability value (P) are depicted on each plot
Discussion
In the current study, CI, SVI, and SVV were continuously monitored with the FloTrac/Vigileo system during surgery. However, fluid resuscitation was managed by relying on conventional hemodynamic indicators including MAP, HR, CVP, urine output, a volume of infusion, and the total intraoperative in–out balance. As a result, there was a wide variation in SVI values at the end of surgery, which was significantly associated with postoperative renal function (Fig. 2). Patients in the low SVI group exhibited a more significant increase in Cr and a more significant decrease in eGFR on POD1 and POD2 compared with those in the normal SVI group. In addition, the incidence of postoperative AKI was higher in the low SVI group than in the normal SVI group. These results suggested that inadequate volume status at the end of esophagectomy indicated by SVI less than 35 ml/m2 could increase the risk of postoperative AKI.
In order to prevent AKI, prompt resuscitation of the circulating blood volume with fluids is the primary aim.6 Avoiding hypovolemia by means of administration of sufficient isotonic crystalloids and maintaining the mean arterial pressure above 60 mm Hg are recommended for the protection of renal function and the prevention of AKI.5,6 During esophagectomy, however, volume status is difficult to assess, because a dynamic and extensive shift of extracellular fluid, which results in depletion of intravascular volume, commonly occurs as a result of extensive surgical manipulation, stress response, and hypercytokinemia.8,9 In the present study, although the total amount of fluid administration and the in–out balance during surgery did not differ between the low and normal SVI groups, the low SVI group had lower SVI at the end of surgery and developed postoperative AKI more frequently. These results suggested that fluid responsiveness varied individually, and that fluid resuscitation therapy should be performed on the basis of some reliable indicator of volume status during esophagectomy. From the results of our study, however, it was clear that conventional hemodynamic indicators such as MAP, HR, CVP, and urine output were insufficient to adequately evaluate volume resuscitation in order to prevent AKI.
A number of studies have shown that SVV and SVI can be more reliable indicators of fluid responsiveness or cardiac preload than static indicators such as CVP and pulmonary artery wedge pressure.22,23 In the present study, SVI was significantly associated with postoperative renal function, suggesting that SVI could be used as a reliable indicator of fluid responsiveness or volume status during esophagectomy. In contrast, SVV was not associated with postoperative renal function. Reportedly, SVV is affected not only by cardiac preload, but also by ventilatory issues (tidal volume, PEEP, chest and lung compliances), whereas SVI is relatively unaffected by these ventilatory issues.24 Therefore, SVV can be used as an indicator of fluid responsiveness only during mechanical ventilation, whereas SVI can be used as a reliable indicator throughout the perioperative period, not only during mechanical ventilation but also during spontaneous breathing.16,24 In the current study, SVV at the end of esophagectomy did not correlate with postoperative renal function, probably because the setting of the ventilator, which could closely affect SVV, was not strictly standardized in our case series, and also because chest and lung compliances, which also could affect SVV, might be altered by varying degrees after major thoracotomy and one-lung ventilation.
A randomized controlled trial reported that an algorithm to maintain an SVI above 35 ml/m2 in the immediate postoperative period after cardiac surgery optimized circulatory status, significantly reduced the rate of complications, and shortened ICU and hospital stay.16 In the present study, we showed that patients with normal SVI were at reduced risk for postoperative AKI. Collectively, these results suggested that a fluid resuscitation strategy to maintain normal SVI (≥35 ml/m2) using the minimally invasive FloTrac/Vigileo system would be effective in protecting renal function and preventing AKI after esophagectomy, though it must be noted that the useful indicators provided by this system is unreliable in patients with severe peripheral arterial constriction, aortic regurgitation, and frequent arrhythmias.8,23
Our results suggested that inadequate volume status at the end of surgery indicated by SVI less than 35 ml/m2 could increase the risk of postoperative AKI in patients undergoing radical esophagectomy. Our results also suggest that fluid resuscitation strategy to maintain normal SVI (≥35 ml/m2) using the minimally invasive FloTrac/Vigileo system would be effective in protecting renal function and preventing AKI after esophagectomy, although this assumption should be verified in a further prospective study.
References
O’Brien MM, Gonzales R, Shroyer AL, et al. Modest serum creatinine elevation affects adverse outcome after general surgery. Kidney Int. 2002;62:585–92.
Mori Y, Sato N, Kobayashi Y, Ochiai R. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth. 2011;25:799–804.
Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–47.
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:31.
Lameire N, Van Biesen W, Vanholder R. Acute kidney injury. Lancet. 2008;372:1863–5.
Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010;36:392–411.
Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116:1139–48.
Kobayashi M, Koh M, Irinoda T, Meguro E, Hayakawa Y, Takagane A. Stroke volume variation as a predictor of intravascular volume depression and possible hypotension during the early postoperative period after esophagectomy. Ann Surg Oncol. 2009;16:1371–7.
Yamaguchi K, Sugasawa Y, Takeuchi K, et al. Effects of sivelestat on bronchial inflammatory responses after esophagectomy. Int J Mol Med. 2011;28:187–92.
Aceto P, Congedo E, Cardone A, Zappia L, De Cosmo G. Postoperative management of elective esophagectomy for cancer. Rays. 2005;30:289–94.
Buise M, Van Bommel J, Mehra M, Tilanus HW, Van Zundert A, Gommers D. Pulmonary morbidity following esophagectomy is decreased after introduction of a multimodal anesthetic regimen. Acta Anaesthesiol Belg. 2008;59:257–61.
Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg. 2003;75:337–41.
Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–20.
Manecke GR. Edwards FloTrac sensor and Vigileo monitor: easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Expert Rev Med Devices. 2005;2:523–7.
Cannesson M, Musard H, Desebbe O, et al. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–7.
McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ. 2004;329:258.
Mailloux PT, Friderici J, Freda B, McGee WT. Establishing goals of volume management in critically ill patients with renal failure. J Nephrol. 2012;25:962–8.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Rutherford BD, McCann WD, O’Donovan TP. The value of monitoring pulmonary artery pressure for early detection of left ventricular failure following myocardial infarction. Circulation. 1971;43:655–66.
Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–95.
Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–43.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–7.
Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101:761–8.
Suehiro K, Okutani R. Influence of tidal volume for stroke volume variation to predict fluid responsiveness in patients undergoing one-lung ventilation. J Anesth. 2011;25:777–80.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugasawa, Y., Hayashida, M., Yamaguchi, K. et al. Usefulness of Stroke Volume Index Obtained with the FloTrac/Vigileo System for the Prediction of Acute Kidney Injury After Radical Esophagectomy. Ann Surg Oncol 20, 3992–3998 (2013). https://doi.org/10.1245/s10434-013-3084-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-3084-5