Abstract
Background
Inflammatory reactions at a tumor site have both detrimental and beneficial effects on tumor progression. This study was designed to assess the clinical significance of tumor-infiltrating inflammatory cells in patients with intrahepatic cholangiocarcinoma (ICC).
Methods
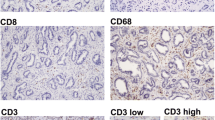
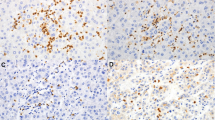
A total of 123 consecutive ICC patients who underwent curative resection were enrolled. Tissue microarray and immunohistochemistry were used to analyze the distribution and clinical relevance of IL-17+, FOXP3+, CD8+, CD66b+ cells, and microvessel density (CD34) in different microanatomical areas.
Results
IL-17+ cells, FOXP3+ lymphocytes, CD66b+ neutrophils, and microvessels were enriched predominantly in intratumor (IT) area, whereas CD8+ lymphocytes were most abundant in tumor invasive front. On univariate analyses, increasing IL-17 +IT and neutrophilsIT were significantly associated with worse patient survival. Multivariate analyses revealed that IL-17 +IT (hazard ratio [HR] = 1.59; 95% confidence interval [CI], 1.05–2.41; P = 0.028), neutrophilsIT (HR = 1.76; 95% CI, 1.16–2.65; P = 0.007), and their combination (HR 2.8; 95% CI 1.72–4.57; P < 0.001) were independent prognostic factors, which were superior to conventional clinicopathologic features, such as intrahepatic metastasis and TNM stage. IL-17 +IT significantly correlated with the presence of lymph node metastasis, intrahepatic metastasis, and advanced stages, whereas neutrophilsIT correlated with the presence of vascular invasion. In addition, significant positive correlations were detected among densities of IL-17+ cells, neutrophils, and microvessel density.
Conclusions
Our data suggested that intratumor IL-17+ cells, neutrophils are novel, powerful predictors of prognosis in patients with ICC.



Similar content being viewed by others
References
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–21.
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O’Reilly E, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96.
Grivennikov S, Greten F, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Gomez D, Morris-Stiff G, Toogood G, Lodge J, Prasad K. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–8.
Sirica A. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15.
Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62.
Crispe I. The liver as a lymphoid organ. Ann Rev Immunol. 2009;27:147-63.
Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, Iijima S, Noike T, Kobayashi A, Kawasaki S. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Human Pathol. 2004;35:881–6.
Shimonishi T, Isse K, Shibata F, Aburatani I, Tsuneyama K, Sabit H, Harada K, Miyazaki K, Nakanuma Y. Up-regulation of Fas ligand at early stages and down-regulation of Fas at progressed stages of intrahepatic cholangiocarcinoma reflect evasion from immune surveillance. Hepatology. 2000;32:761–9.
Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7‐H1 on intrahepatic cholangiocarcinoma cells with PD‐1 on tumor‐infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–4.
Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–9.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586–93.
Yakirevich E, Resnick MB. Regulatory T lymphocytes: pivotal components of the host antitumor response. J Clin Oncol 2007;25:2506–8.
Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 2010;10:248–56.
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis 2011 32:643–9.
Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2010;26:269–73.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–9.
Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 2010;51:154–64.
Laconi E. The evolving concept of tumor microenvironments. Bioessays 2007;29:738–44.
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009;114:1141–9.
fanos K, Bruno T, Maris C, Xu L, Thoburn C, DeMarzo A, Meeker A, Isaacs W, Drake C. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 2008; 14:3254–61.
Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One 2011;6:e18219.
Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011;71:1263–71.
Murugaiyan G, Saha B. Protumor vs. antitumor functions of IL-17. J Immunol 2009;183:4169–75.
Kuang D, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin X, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 2011;54:948–55.
Jensen H, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009;27:4709–17.
Li Y, Qiu S, Fan J, Zhou J, Gao Q, Xiao Y, Xu Y. Intratumoral neutrophils as poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497–505.
Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–82.
Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell 2009;16:173–4.
Shojaei F, Singh M, Thompson J, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA 2008;105:2640-5.
Murdoch C, Muthana M, Coffelt S, Lewis C. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44.
Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr Opin Oncol 2009;21:53–9.
Acknowledgment
This study was supported by research grants from the State 863 High Technology R&D Project of China (No. 2007AA02Z479), FANEDD (for Q.G.), National Natural Science Foundation of China (No. 30901432, 30972949), Shanghai Rising-Star Program (No. 10QA1401300), and “Chen Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 11CG02).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
F.-M. Gu, Q. Gao, G.-M. Shi, and X. Zhang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2012_2268_MOESM2_ESM.tif
Supplementary Fig. 1 Representative distribution of IL-17, CD66b, FOXP3, CD34, and CD8-positive cells in different microanatomic areas of ICC samples (n = 16). The micrographs at higher magnification show the stained intratumor area (1), invasive edge (2), and noncancerous peritumor area (3). Except that CD8+ cells were mainly enriched in the invasive edge, the remaining types of cells most often were found in the intratumor area. The area between the two dashed lines denotes tumor invasive front (TIFF 7808 kb)
Rights and permissions
About this article
Cite this article
Gu, FM., Gao, Q., Shi, GM. et al. Intratumoral IL-17+ Cells and Neutrophils show Strong Prognostic Significance in Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 19, 2506–2514 (2012). https://doi.org/10.1245/s10434-012-2268-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2268-8