Abstract
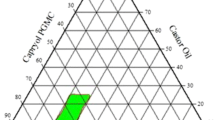
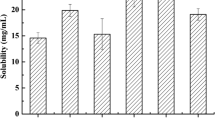
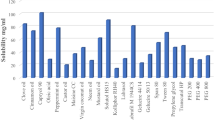
This work aimed to enhance the oral bioavailability of diacerein. The drug was incorporated in self-nanoemulsifying drug delivery system. Ternary phase diagrams were constructed using Capryol™90, Miglyol®812 and isopropyl myristate as oils, Tween®80 and Tween®20 as surfactants and PEG 200 and PEG 300 as co-surfactants. Among a total of 432 formulae, 17 formulae were clear. They were assessed for mean droplet size, polydispersity index (PDI), saturation solubility and transmission electron microscopy. Solid granules were obtained by adsorption on Aeroperl®300. Results for DSC, PXRD, and SEM of prepared granules revealed that diacerein was molecularly dispersed within the formula. Desirability factor was adopted to find the granules with maximum solubility, maximum dissolution efficiency, maximum dissolution rate and percentage of drug dissolved at 5 min and minimum dissolution time and Carr’s index. The optimized formula consisted of 10% Miglyol®812, 70% Tween®80 and 20% PEG 200 adsorbed to Aeroperl® 300 with a ratio of 2:1 preconcentrate:carrier. It recorded a 3.77-fold increase in bioavailability, compared to the marketed product. Such enhancement means lower doses and less gastrointestinal side effects.








Similar content being viewed by others
Abbreviations
- BCS:
-
Biopharmaceutics Classification System
- SNEDDS:
-
Self-nanoemulsifying drug delivery systems
- SNEG:
-
Self-nanoemulsifying granules
- MDS:
-
Mean droplet size
- PDI:
-
Polydispersity index
- TEM:
-
Transmission electron microscopy
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
Fourier transform infrared spectroscopy
- PXRD:
-
Powder X-ray diffraction
- SEM:
-
Scanning electron microscopy
- D.E.0–30 :
-
Dissolution efficiency 30 min
- MDT:
-
Mean dissolution time
- Q 5 :
-
Percentage of drug released at time 5 min
- DR5 :
-
Dissolution rate during first 5 min of dissolution
- REC:
-
Research Ethics Committee
- LC-MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- IS:
-
Internal standard
- HLB:
-
Hydrophilic–lipophilic balance
- IPM:
-
Isopropyl myristate
References
Elsayed I, Abdelbary AA, Elshafeey AH. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomedicine. 2014;9:2943.
El-Laithy HM, Basalious EB, El-Hoseiny BM, Adel MM. Novel self-nanoemulsifying self-nanosuspension (SNESNS) for enhancing oral bioavailability of diacerein: simultaneous portal blood absorption and lymphatic delivery. Int J Pharm. 2015;490(1):146–54.
Maski N, Kumaran A, Girhepunje K, Ghode P, Randive S, Pal R. Studies on the preparation, characterization and solubility of β-cyclodextrin-diacerein inclusion complexes. Int J Pharm Pharm Sci. 2009;1(2):121–35.
Jain A, Singh SK, Singh Y, Singh S. Development of lipid nanoparticles of diacerein, an antiosteoarthritic drug for enhancement in bioavailability and reduction in its side effects. J Biomed Nanotechnol. 2013;9(5):891–900.
Aggarwal AK, Singh S. Physicochemical characterization and dissolution study of solid dispersions of diacerein with polyethylene glycol 6000. Drug Dev Ind Pharm. 2011;37(10):1181–91.
Abdelbary AA, Elshafeey AH, El-Nabarawi M, Elassasy A, Li X, Jasti B. Comparative in vivo evaluation of aripiprazole coprecipitate, nanoparticles and marketed tablets in healthy human volunteers and in vitro-in vivo correlation. Curr Trends Biotechnol Pharm. 2011;5(4):1397–409.
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–82.
Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–616.
Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro and in vivo evaluation. J Mol Liq. 2016;218:219–32.
Tang B, Cheng G, Gu J-C, Xu C-H. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov Today. 2008;13(13):606–12.
Soliman KA, Ibrahim HK, Ghorab MM. Formulation of avanafil in a solid self-nanoemulsifying drug delivery system for enhanced oral delivery. Eur J Pharm Sci. 2016;93:447–55.
El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355(1):285–92.
Radwan SAA, ElMeshad AN, Shoukri RA. Microemulsion loaded hydrogel as a promising vehicle for dermal delivery of the antifungal sertaconazole: design, optimization and ex vivo evaluation. Drug Dev Ind Pharm. 2017;43(8):1351–65.
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm. 2010;391(1):203–11.
Xue X, Cao M, Ren L, Qian Y, Chen G. Preparation and optimization of rivaroxaban by self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability and no food effect. AAPS PharmSciTech. 2018;19(4):1847–59.
Jain A, Kaur R, Beg S, Kushwah V, Jain S, Singh B. Novel cationic supersaturable nanomicellar systems of raloxifene hydrochloride with enhanced biopharmaceutical attributes. Drug Deliv Transl Res. 2018:1–23.
Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res. 2008;31(8):1040–9.
Patil SB, Shete DK, Narade SB, Surve SS, Khan ZK, Bhise SB, et al. Improvement in the dissolution profile of diacerein using a surfactant-based solid dispersion technique. Drug Discov Ther. 2010;4(6):435–41.
Khan K. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27(1):48–9.
Ibrahim HK, Fahmy RH. Localized rosuvastatin via implantable bioerodible sponge and its potential role in augmenting bone healing and regeneration. Drug Deliv. 2016;23(9):3181–92.
Derringer G, Suich R. Simultaneous optimization of several response variables. J Qual Technol. 1980;12(4):214–9.
Pathak A, Rajput SJ. Diacerein nanosuspension: process optimization, physicochemical characterization, cytotoxicity assessment and in-vivo evaluation for oral bioavailability enhancement. Pharm Res. 2016;6(10):6595–615.
Volpato NM, Silva RL, Brito APP, Gonçalves JCS, Vaisman M, Noël F. Multiple level C in vitro/in vivo correlation of dissolution profiles of two l-thyroxine tablets with pharmacokinetics data obtained from patients treated for hypothyroidism. Eur J Pharm Sci. 2004;21(5):655–60.
Aziz DE, Abdelbary AA, Elassasy AI. Fabrication of novel elastosomes for boosting the transdermal delivery of diacerein: statistical optimization, ex-vivo permeation, in-vivo skin deposition and pharmacokinetic assessment compared to oral formulation. Drug Deliv. 2018;25(1):815–26.
Shrivastava AR, Ursekar B, Kapadia CJ. Design, optimization, preparation and evaluation of dispersion granules of valsartan and formulation into tablets. Curr Drug Deliv. 2009;6(1):28–37.
Khetarpal NA, Ramachal AKS, Rao L, Amin PD. Formulation development of a stable solid oral dosage form of Valproic acid using colloidal silica. Int J Drug Deliv. 2012;4(2):266.
Sandhya S, Gowthami G, Vinod K, VidyaSravanthi E, Saikumar P, Rao K. Formulation and evaluation of herbal effervescent granules incorporated with Limnophila indica extract for bacillary dysentery. Ann Bio Res. 2012;3(1):63–72.
Nasr A, Gardouh A, Ghorab M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8(3):20.
Balakrishnan P, Lee B-J, Oh DH, Kim JO, Hong MJ, Jee J-P, et al. Enhanced oral bioavailability of dexibuprofen by a novel solid self-emulsifying drug delivery system (SEDDS). Eur J Pharm Biopharm. 2009;72(3):539–45.
El-Laithy HM. Self-nanoemulsifying drug delivery system for enhanced bioavailability and improved hepatoprotective activity of biphenyl dimethyl dicarboxylate. Curr Drug Deliv. 2008;5(3):170–6.
Nekkanti V, Karatgi P, Prabhu R, Pillai R. Solid self-microemulsifying formulation for candesartan cilexetil. AAPS PharmSciTech. 2010;11(1):9–17.
Zhao Y, Wang C, Chow AH, Ren K, Gong T, Zhang Z, et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2010;383(1):170–7.
Constantinides PP, Scalart J-P, Lancaster C, Marcello J, Marks G, Ellens H, et al. Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides. Pharm Res. 1994;11(10):1385–90.
Singh AK, Chaurasiya A, Awasthi A, Mishra G, Asati D, Khar RK, et al. Oral bioavailability enhancement of exemestane from self-microemulsifying drug delivery system (SMEDDS). AAPS PharmSciTech. 2009;10(3):906–16.
Elnaggar YS, El-Massik MA, Abdallah OY. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm. 2009;380(1):133–41.
Morey TE, Modell JH, Shekhawat D, Grand T, Shah DO, Gravenstein N, et al. Preparation and anesthetic properties of propofol microemulsions in rats. J Am Soc Anesthesiol. 2006;104(6):1184–90.
Attwood D, Mallon C, Ktistis G, Taylor C. A study on factors influencing the droplet size in nonionic oil-in-water microemulsions. Int J Pharm. 1992;88(1):417–22.
Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. J Control Release. 2002;81(1):65–74.
Gao Z-G. Thermal reversible microemulsion for oral delivery of poorly water-soluble drugs. In: Thermal reversible microemulsion for oral delivery of poorly water-soluble drugs: INTECH open access publisher; 2012.
Chavda H, Patel J, Chavada G, Dave S, Patel A, Patel C. Self-nanoemulsifying powder of isotretinoin: preparation and characterization. J Powder Technol. 2013;2013:1–9.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61.
Kang JH, Oh DH, Oh Y-K, Yong CS, Choi H-G. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur J Pharm Biopharm. 2012;80(2):289–97.
Vander Kloet J, Schramm LL, Shelfantook B. Application of the hydrophile–lipophile balance concept to the classification of demulsifiers and bituminous froth and its components. Fuel Process Technol. 2002;75(1):9–26.
Shanmugam S, Baskaran R, Balakrishnan P, Thapa P, Yong CS, Yoo BK. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur J Pharm Biopharm. 2011;79(2):250–7.
Planinšek O, Kovačič B, Vrečer F. Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int J Pharm. 2011;406(1):41–8.
Beg S, Swain S, Singh HP, Patra CN, Rao MB. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech. 2012;13(4):1416–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiment protocol was accepted by the Research Ethics Committee of Faculty of Pharmacy, Cairo University, Cairo, Egypt (PI 911).
Conflict of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Naseef, M.A., Ibrahim, H.K. & Nour, S.A.EK. Solid Form of Lipid-Based Self-Nanoemulsifying Drug Delivery Systems for Minimization of Diacerein Adverse Effects: Development and Bioequivalence Evaluation in Albino Rabbits. AAPS PharmSciTech 19, 3097–3109 (2018). https://doi.org/10.1208/s12249-018-1138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1138-5