Abstract
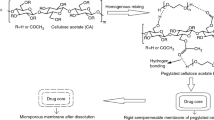
Micro/nanoporous osmotic pump tablets coated with cellulose acetate containing polyvinylpyrolidone (PVP) as pore formers were fabricated. Propranolol hydrochloride was used as a model drug in this study. Formulation optimization based on USP 31 requirements was conducted following a central composite design using a two-level factorial plan involving two membrane variables (pore former and coating levels). Effect of molecular weight of pore former (PVP K30 and PVP K90) was also evaluated. Responses of drug release to the variables were analyzed using statistical software (MINITAB 14). Scanning electron microscopy and atomic force microscopy showed that the pores formed by PVP. The drug release was dependent on the molecular weight and concentration of PVP and the level of coating. The results showed that acceptable 12-h profile could be achieved with only specific range of PVP K30-containing membrane at the defined membrane thickness. However, satisfactory 24-h profile could be accomplished by both PVP K30 and PVP K90-containing membrane at the range and membrane thickness tested. Preparation and testing of the optimized formulation showed a good correlation between predicted and observed values.









Similar content being viewed by others
REFERENCES
Theeuwes F. Elementary osmotic pump. J Pharm Sci. 1975;64(12):1987–91.
Wong PSL, Gupta SK, Stewart BE. Osmotically controlled tablets. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified-release drug delivery technology. New York: Marcel Dekker; 2003. p. 101–14.
Ramakrishna N, Mishra B. Plasticizer effect and comparative evaluation of cellulose acetate and ethylcellulose-HPMC combination coatings as semipermeable membranes for oral osmotic pumps of naproxen sodium. Drug Dev Ind Pharm. 2002;28(4):403–12.
Rani M, Surana R, Sankar C, Mishra B. Development and biopharmaceutical evaluation of osmotic pump tablets for controlled delivery of diclofenac sodium. Acta Pharm. 2003;53:263–73.
Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm. 2004;57:513–25.
Zentner GM, Rork GS, Himmelstein KJ. The controlled porosity osmotic pump. J Control Release. 1985;1(4):269–82.
Zentner GM, Rork GS, Himmelstein KJ. Osmotic flow through controlled porosity films: an approach to delivery of water soluble compounds. J Control Release. 1985;2:217–29.
Santus G, Baker RW. Osmotic drug delivery: review of the patent literature. J Control Release. 1995;35:1–21.
Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Control Release. 2002;79(1–3):7–27.
Appel LE, Zentner GM. Use of modified ethylcellulose lattices for microporous coating of osmotic tablets. Pharm Res. 1991;8(5):600–4.
Okimoto K, Tokunaga Y, Ibuki R, Irie T, Uekama K, Rajewski RA, et al. Applicability of (SBE)7 m-beta-CD in controlled-porosity osmotic pump tablets (OPTs). Int J Pharm. 2004;286(1–2):81–8.
Zentner GM, Rork GS, Himmelstein KJ, inventors; Merck & Co., Inc., assignee. Controlled porosity osmotic pump. US patent 4,880,631. November 6, 1990.
McClelland GA, Sutton SC, Engle K, Zentner GM. The solubility-modulated osmotic pump: in vitro/in vivo release of diltiazem hydrochloride. Pharm Res. 1991;8:88–92.
Verma RK, Kaushal AM, Garg S. Development and evaluation of extended release formulations of isosorbide mononitrate based on osmotic technology. Int J Pharm. 2003;263:9–24.
Wong PSL, Barclay B, Deters JC, Theeuwes F, inventors; Alza Corporation, assignee. Osmotic device with dual thermodynamic activity. US patent 4,612,008. September 16, 1986.
Ozdemir N, Sahin J. Design of a controlled release osmotic pump system of ibuprofen. Int J Pharm. 1997;158(1):91–7.
Lin S-Y, K-h L, Li M-J. Influence of excipients, drugs, and osmotic agent in the inner core on the time-controlled disintegration of compression-coated ethylcellulose tablets. J Pharm Sci. 2002;91(9):2040–6.
Zhang Y, Zhang Z, Wu F. A novel pulsed-release system based on swelling and osmotic pumping mechanism. J Control Release. 2003;89(1):47–55.
He F, MacGregor G. How far should salt intake be reduced? Hypertension. 2003;42(6):1093–9.
He F, MacGregor G. Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol. 2007;22(4):298–305.
Schwartz JB, O'Connor RE, Schnaare RL. Optimization techniques in pharmaceutical formulation and processing. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker; 2002. p. 607–26.
Myer RH, Montgomery DC. Response surface methodology. 2nd ed. New York: Wiley; 2002.
U.S. Department of Health and Human Service Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry: Modified release solid oral dosage forms: SUPAC-MR: Chemistry, manufacturing and controls, in vitro dissolution testing and in vivo bioequivalence documentation. September 1997. http://www.fda.gov/cder/guidance/1214fnl.pdf. Accessed Sept 5, 2008.
O'Hara T, Dunne A, Butler J, Devane J. A review of methods used to compare dissolution profile data. Pharm Sci Tech Today. 1998;1(5):214–23.
Gil EC, Colarte AI, Sampedro JLL, Bataille B. Subcoating with Kollidon VA64 as water barrier in a new combined native dextran/HPMC-cetyl alcohol controlled release tablet. Eur J Pharm Biopharm. 2008;69:303–11.
Shanghai Sunpower Material. PVP. http://www.chinapvp.com/technoinfo-1.htm. Accessed Sept 5, 2008.
Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. 4th ed. London: Pharmaceutical Press; 2003.
Okimoto K, Ohike A, Ibuki R, Aoki O, Ohnishi N, Rajewski RA, et al. Factors affecting membrane-controlled drug release for an osmotic pump tablet (OPT) utilizing (SBE)7 m-beta-CD as both a solubilizer and osmotic agent. J Control Release. 1999;60(2–3):311–19.
Kim YK, Park HB, Lee YM. Gas separation properties of carbon molecular sieve membranes derived from polyimide/polyvinylpyrrolidone blends: effect of the molecular weight of polyvinylpyrrolidone. J Membrane Sci. 2005;251:159–67.
De Muth JE. Basic statistics and pharmaceutical statistical applications. 2nd ed. New York: Chapman & Hall; 2006.
Dietrich P, Bauer-Brandi A, Schubert R. Influence of tableting forces and lubricant concentration on the adhesion strength in complex layer tablets. Drug Dev Ind Pharm. 2000;26:745–54.
Montgomery DC. Design and analysis of experiments. 4th ed. New York: Wiley; 1997.
Fritzsche AK, Arevalo AR, Moore MD, Elings VB, Kjoller K, Wu CM. The surface structure and morphology of polyvinylidenefluoride microfiltration membranes by atomic force microscopy. J Membr Sci. 1992;68:65–78.
Dietz P, Hansma PK, Inacker O, Lehmann H, Herrmann K. Surface pore structures of micro- and ultrafiltration membranes imaged with the atomic force microscope. J Membr Sci. 1992;65:101–11.
Palacio L, Pradanos P, Calvo JI, Hernandez A. Porosity measurements by a gas penetration method and other techniques applied to membrane characterization. Thin Solid Films. 1999;348:22–9.
Kim JH, Min BR, Park HC, Won J, Kang YS. Phase behavior and morphological studies of polyimide/PVP/solvent/water system by phase inversion. J Appl Polym Sci. 2001;81:3481–8.
Ochoa NA, Pradanos P, Palacio L, Pagliero C, Marchese J, Hernandez A. Pore size distributions based on AFM imaging and retention of multidisperse polymer solutes: characterisation of polyethersulfone UF membranes with dopes containing different PVP. J Membr Sci. 2001;187:227–37.
Kim YK, Park HB, Lee YM. Carbon molecular sieve membrane derived from thermally labile polymer containing blend polymers and their gas separation properties. J Membrane Sci. 2004;243:9–17.
Nakao S. Determination of pore size and pore size distribution 3.Filtration membranes. J Membr Sci. 1994;96:131–65.
ACKNOWLEDGMENT
Financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0095/2544) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuntikulwattana, S., Mitrevej, A., Kerdcharoen, T. et al. Development and Optimization of Micro/Nanoporous Osmotic Pump Tablets. AAPS PharmSciTech 11, 924–935 (2010). https://doi.org/10.1208/s12249-010-9446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9446-4