Abstract
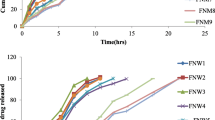
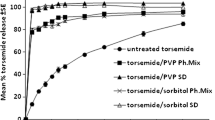
The purpose of this research was to prepare and evaluate sustained release mucoadhesive tablets of Itraconazole. It is practically insoluble in aqueous fluids hence its solid dispersion with Eudragit E100 was prepared by spray drying. This was formulated in matrix of hydrophilic mucoadhesive polymers Carbopol 934P (CP) and Methocel K4M (HPMC). The formulation was optimized using a 32 factorial design. Amounts of CP and HPMC were taken as formulation variables for optimizing response variables i.e. mucoadhesion and dissolution parameters. The optimized mucoadhesive formulation was orally administered to albino rabbits, and blood samples collected were used to determine pharmacokinetic parameters. The solid dispersion markedly enhanced the dissolution rate of itraconazole. The bioadhesive strength of formulation was found to vary linearly with increasing amount of both polymers. Formulations exhibited drug release fitting Peppas model with value of n ranging from 0.61 to 1.18. Optimum combination of polymers was arrived at which provided adequate bioadhesive strength and fairly regulated release profile. The experimental and predicted results for optimum formulations were found to be in close agreement. The formulation showed C max 1898 ± 75.23 ng/ml, t max of the formulation was 2 h and AUC was observed to be 28604.9 ng h/ml









Similar content being viewed by others
References
S. Grant, and S. Clissold. Itraconazole: a review of pharmacodynamics and pharmacokinetic properties and therapeutic use in superficial and systemic mycosis. Ind. Drugs. 37:310–314 (1989).
J. Jacob, M. Bassett, B. Carter, et. al. Pharmacokinetics of bioadhesive, gastroretentive, controlled release tablets of itraconazole. (2005) Accessed at: http://www.spherics.com
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutics drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
A. T. M. Serajuddin. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 88:1058–1066 (1999).
J. S. Woo and H. G. Yi. Antifungal oral composition containing itraconazole and process for preparing same. US Patent 6,039,981, March (2000).
J. Peeters, P. Neeskens, J. P. Tollenaere, P. Van Remoortere, and M. Brewster. Characterization of the interaction of 2-hydroxypropyl-b-cyclodextrin with itraconazole at pH 2, 4 and 7. J. Pharm. Sci. 91:1414–1422 (2002).
K. Six, T. Daems, J. de Hoon, A. V. Hecken, M. Depre, M. P. Bouche, P. Prinsen, G. Verreck, J. Peeters, M. E. Brewster, and G. V. den Mooter. Clinical study of solid dispersions of itraconazole prepared by hot-stage extrusion. Eur. J. Pharm. Sci. 24:179–186 (2005).
B. Rambali, G. Verreck, L. Baert, and D. L. Massart. Itraconazole formulation studies of the melt-extrusion process with mixture design. Drug Dev. Ind. Pharm. 29:641–652 (2003).
G. Stetsko. Statistical experimental design and its application to pharmaceutical development problems. Drug Dev. Ind. Pharm. 12:1109–1123 (1986).
S. Dawoodbhai, E. R. Suryanarayan, C. W. Woodruff, and C. T. Rhodes. Optimization of tablet formulations containing talc. Drug Dev Ind Pharm. 17(10):1343–1371 (1991).
C. E. Bos, G. S. Bolhuis, and C. F. Lerk. Optimization of tablet formulations based on starch/lactose granulations for use in tropical countries. Drug Dev. Ind. Pharm. 17(17):2373–2389 (1991).
G. C. Ceschel, P. Maffei, and R. Badiello. Optimization of hydrochlorothiazide tablets. Drug Dev Ind Pharm. 25(11):1167–1176 (1999).
Jung et al. Method and composition of an oral preparation of itraconazole. United States Patent 6 485 743. November 26 (2002).
A. Gupta, S. Garg, and R. K. Khar. Measurement of bioadhesive strength of mucoadhesive buccal tablets: design of an in vitro assembly. Indian Drugs. 30(4):152–155 (1993).
C. Paulo, and J. M. S. Lobo. Modeling and comparison of dissolution profiles. Eur. J. Pharm Sci. 13:123–133 (2001).
S. D. Yoo, S. H. Lee, E. Kang, H. Jun, J. Y. Jung, J. W. Park, and K. H. Lee. Bioavailability of itraconazole in rats and rabbits after administration of tablets containing solid dispersion particles. Drug Dev. Ind. Pharm. 26(1):27–34 (2000).
K. Six, Ch. Leuner, J. Dressman, G. Verreck, J. Peeters, N. Blaton, P. Augustijns, R. Kinget, and G. Van den Mooter. Thermal properties of hot-stage extrudates of itraconazole and eudragit E100. Phase separation and polymorphism. J. Therm. Anal. Calorim. 68(2):591–601 (2000).
K. G. H. Goud, and T. M. P. Kumar. Preparation and evaluation of a novel buccal adhesive system. AAPS PharmSciTech. 5(3):article 35 (2004).
K. Satoh, K. Takayama, Y. Machida, Y. Suzuki, M. Nakagaki, and T. Nagai. Factors affecting the bioadhesive property of tablets consisting of hydroxypropyl cellulose and carboxyvinyl polymer. Chem. Pharm. Bull. 37(5):1366–1368 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madgulkar, A., Kadam, S. & Pokharkar, V. Studies on Formulation Development of Mucoadhesive Sustained Release Itraconazole Tablet Using Response Surface Methodology. AAPS PharmSciTech 9, 998–1005 (2008). https://doi.org/10.1208/s12249-008-9119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9119-8