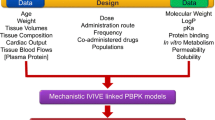
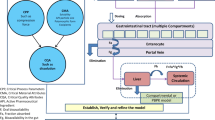
Abstract
Model informed drug development (MiDD) is useful to predict in vivo exposure of drugs during various stages of the drug development process. This approach employs a variety of quantitative tools to assess the risks during the drug development process. One important tool in the MiDD tool kit is the Physiologically Based Pharmacokinetic Modelling (PBPK). This tool is extensively used to reduce the development cost and to accelerate the access of medicines to the patients. In this work, we provide an overview of PBPK modelling approaches in the generic drug development process, with a special emphasis on the bio-waiver applications. We describe herein approaches and common pitfalls while submitting model based justifications as a response to the regulatory deficiencies during the generic drug development process. With some in-house case studies, we have attempted to provide a clear path for PBPK model based justifications for bio-waivers. With this review, the gap between theoretical knowledge and practical application of modelling and simulation tools for generic drug product development could be potentially reduced.
Graphical abstract







Similar content being viewed by others
References
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharm Ther. 2011;89(2):259–67. https://doi.org/10.1038/clpt.2010.298.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37. https://doi.org/10.1124/dmd.115.065920.
El-Khateeb E, Burkhill S, Murby S, Amirat H, Rostami-Hodjegan A, Ahmad A. Physiological-based pharmacokinetic modeling trends in pharmaceutical drug development over the last 20-years; in-depth analysis of applications, organizations, and platforms. Biopharm Drug Dispos. 2021;42(4):107–17. https://doi.org/10.1002/bdd.2257.
Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. Committee for Medicinal Products for Human Use (CHMP). 2018; Available from:https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf. Accessed on 15 Dec 2022
Physiologically based pharmacokinetic analyses — format and content. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2018. https://www.fda.gov/media/101469/download. Accessed on 15 Dec 2022
Laisney M, Heimbach T, Mueller-Zsigmondy M, Blumenstein L, Costa R, Ji Y. Physiologically based biopharmaceutics modeling to demonstrate virtual bioequivalence and bioequivalence safe-space for ribociclib which has permeation rate-controlled absorption. J Pharm Sci. 2022 111(1):274-284. https://doi.org/10.1016/j.xphs.2021.10.017.
Wu D, Sanghavi M, Kollipara S, Ahmed T, Saini AK, Heimbach T. Physiologically based pharmacokinetics modeling in biopharmaceutics: case studies for establishing the bioequivalence safe space for innovator and generic drugs. Pharm Res. 2023;40(2):337–57. https://doi.org/10.1007/s11095-022-03319-6.
Shebley M, Sandhu P, Emami Riedmaier A, Jamei M, Narayanan R, Patel A, Peters SA, Reddy VP, Zheng M, de Zwart L, Beneton M. Physiologically based pharmacokinetic model qualification and reporting procedures for regulatory submissions: a consortium perspective. Clin Pharm Ther. 2018;104(1):88–110. https://doi.org/10.1002/cpt.1013.
Naga D, Parrott N, Ecker GF, Olivares-Morales A. Evaluation of the success of high-throughput physiologically based pharmacokinetic (HT-PBPK) modeling predictions to inform early drug discovery. Mol Pharm. 2022;19(7):2203–16. https://doi.org/10.1021/acs.molpharmaceut.2c00040.
Kilford PJ, Chen KF, Crewe K, Gardner I, Hatley O, Ke AB, Neuhoff S, Zhang M, Rowland YK. Prediction of CYP-mediated DDIs involving inhibition: Approaches to address the requirements for system qualification of the Simcyp Simulator. CPT: Pharmacometrics & Systems. Pharmacol. 2022;11(7):822–32. https://doi.org/10.1002/psp4.12794.
Hanke N, Frechen S, Moj D, Britz H, Eissing T, Wendl T, Lehr T. PBPK models for CYP3A4 and P-gp DDI prediction: a modeling network of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin. CPT: Pharm Syst Pharm. 2018;7(10):647–59. https://doi.org/10.1002/psp4.12343.
Frechen S, Solodenko J, Wendl T, Dallmann A, Ince I, Lehr T, Lippert J, Burghaus R. A generic framework for the physiologically-based pharmacokinetic platform qualification of PK-Sim and its application to predicting cytochrome P450 3A4–mediated drug–drug interactions. CPT: Pharm Syst Pharmacol. 2021;10(6):633–44. https://doi.org/10.1002/psp4.12636.
Loisios-Konstantinidis I, Dressman J. Physiologically based pharmacokinetic/pharmacodynamic modeling to support waivers of in vivo clinical studies: current status, challenges, and opportunities. Mol Pharm. 2020;18(1):1–7. https://doi.org/10.1021/acs.molpharmaceut.0c00903.
Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, Block M, Eissing T, Teutonico D. Applied concepts in PBPK modeling: how to build a PBPK/PD model. CPT: Pharm Syst Pharmacol. 2016;5(10):516–31. https://doi.org/10.1002/psp4.12134.
Advancing biopharmaceutics & drug formulation using in silico modeling model-informed formulation development (MIFD) increases speed and certainty for new and generic drugs; Available from: https://www.certara.com/app/uploads/2023/03/WP_Model-Informed-Formulation-Development_final.pdf. Accessed on 30th march 2023
CDER Conversation: Model Informed Drug Development 2018; Available from: https://www.fda.gov/drugs/news-events-human-drugs/cder-conversation-model-informed-drug-development. Accessed on 15 Dec 2022
Wu F, Shah H, Li M, Duan P, Zhao P, Suarez S, Raines K, Zhao Y, Wang M, Lin HP, Duan J. Biopharmaceutics applications of physiologically based pharmacokinetic absorption modeling and simulation in regulatory submissions to the US food and drug administration for new drugs. The AAPS J. 2021;23:1–4. https://doi.org/10.1208/s12248-021-00564-2.
Anand O, Pepin XJ, Kolhatkar V, Seo P. The use of physiologically based pharmacokinetic analyses—in biopharmaceutics applications-regulatory and industry perspectives. Pharm Res. 2022;39(8):1681–700. https://doi.org/10.1007/s11095-022-03280-4.
The use of physiologically based pharmacokinetic analyses — biopharmaceutics applications for oral drug product development, manufacturing changes, and controls guidance for industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2020; Available from: https://www.fda.gov/media/142500/download. Accessed on 15 Dec 2022
Loisios-Konstantinidis I, Hens B, Mitra A, Kim S, Chiann C, Cristofoletti R. Using physiologically based pharmacokinetic modeling to assess the risks of failing bioequivalence criteria: a tale of two ibuprofen products. The AAPS J. 2020;22:1–9. https://doi.org/10.1208/s12248-020-00495-4.
Jaiswal S, Ahmed T, Kollipara S, Bhargava M, Chachad S. Development, validation and application of physiologically based biopharmaceutics model to justify the change in dissolution specifications for DRL ABC extended release tablets. Drug Dev Indust Pharma. 2021;47(5):778–89. https://doi.org/10.1080/03639045.2021.1934870.
Willmann S, Thelen K, Lippert J. Integration of dissolution into physiologically-based pharmacokinetic models III: PK-Sim®. J Pharma Pharmacol. 2012;64(7):997–1007. https://doi.org/10.1111/j.2042-7158.2012.01534.x.
Dong Z, Li J, Wu F, Zhao P, Lee SC, Zhang L, Seo P, Zhang L. Application of physiologically-based pharmacokinetic modeling to predict gastric pH-dependent drug–drug interactions for weak base drugs. CPT: Pharmacom Syst Pharmacol. 2020;9(8):456–65. https://doi.org/10.1002/psp4.12541.
Chirumamilla SK, Banala VT, Jamei M, Turner DB. Mechanistic PBPK modelling to predict the advantage of the salt form of a drug when dosed with acid reducing agents. Pharm. 2021;13(8):1169. https://doi.org/10.3390/pharmaceutics13081169.
Gray VA, Mann JC, Barker R, Pepin XJ. The case for physiologically based biopharmaceutics modelling (PBBM): what do dissolution scientists need to know. Dev. 2020;12:14. https://doi.org/10.14227/DT270320P6.
Mitra A, Parrott N, Miller N, Lloyd R, Tistaert C, Heimbach T, Ji Y, Kesisoglou F. Prediction of pH-dependent drug-drug interactions for basic drugs using physiologically based biopharmaceutics modeling: industry case studies. J Pharm Sci. 2020;109(3):1380–94. https://doi.org/10.1016/j.xphs.2019.11.017.
Evaluation of gastric pH dependent drug interactions with acid-reducing agents: study design, data analysis, and clinical implications. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2020; Available from: https://www.fda.gov/media/144026/download. Accessed on 15 Dec 2022
Cheng L, Wong H. Food effects on oral drug absorption: application of physiologically-based pharmacokinetic modeling as a predictive tool. Pharm. 2020;12(7):672. https://doi.org/10.3390/pharmaceutics12070672.
Li M, Zhao P, Pan Y, Wagner C. Predictive performance of physiologically based pharmacokinetic models for the effect of food on oral drug absorption: current status. CPT: Pharm Syst Pharmacol. 2018;7(2):82–9. https://doi.org/10.1002/psp4.12260.
Riedmaier AE, Lindley DJ, Hall JA, Castleberry S, Slade RT, Stuart P, Carr RA, Borchardt TB, Bow DA, Nijsen M. Mechanistic physiologically based pharmacokinetic modeling of the dissolution and food effect of a biopharmaceutics classification system IV compound—the venetoclax story. J Pharm Sci. 2018;107(1):495–502. https://doi.org/10.1016/j.xphs.2017.09.027.
Andreas CJ, Pepin X, Markopoulos C, Vertzoni M, Reppas C, Dressman JB. Mechanistic investigation of the negative food effect of modified release zolpidem. Eur J Pharm Sci. 2017;1(102):284–98. https://doi.org/10.1016/j.ejps.2017.03.011.
Rebeka J, Jerneja O, Igor L, Boštjan P, Aleksander B, Simon Ž, Albin K. PBPK absorption modeling of food effect and bioequivalence in fed state for two formulations with crystalline and amorphous forms of BCS 2 class drug in generic drug development. AAPS PharmSciTech. 2019 20:1-0. https://doi.org/10.1208/s12249-018-1285-8.
Dodd S, Kollipara S, Sanchez-Felix M, Kim H, Meng Q, Beato S, Heimbach T. Prediction of ARA/PPI drug-drug interactions at the drug discovery and development interface. J Pharm Sci. 2019;108(1):87–101. https://doi.org/10.1016/j.xphs.2018.10.032.
Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug–drug interactions for weak base drugs: potential implications for new drug development. Clin Pharm Ther. 2014;96(2):266–77. https://doi.org/10.1038/clpt.2014.87.
Bhattachar SN, Perkins EJ, Tan JS, Burns LJ. Effect of gastric pH on the pharmacokinetics of a bcs class II compound in dogs: utilization of an artificial stomach and duodenum dissolution model and gastroplus,™ simulations to predict absorption. J Pharm Sci. 2011;100(11):4756–65. https://doi.org/10.1002/jps.22669.
Gray VA, Diaz DA, Dressman J, Tsume Y, Fotaki N. Highlights from the 2020 AAPS 360 Annual Meeting. Dissolution Technol. 2021;28(2):36–41. https://doi.org/10.14227/DT280221P36.
Mittapelly N, Polak S. Modelling and simulation approaches to support formulation optimization, clinical development and regulatory assessment of the topically applied formulations–Nimesulide solution gel case study. Eur J Pharm Biopharm. 2022;1(178):140–9. https://doi.org/10.1016/j.ejpb.2022.08.005.
Tsakalozou E, Babiskin A, Zhao L. Physiologically-based pharmacokinetic modeling to support bioequivalence and approval of generic products: a case for diclofenac sodium topical gel, 1%. CPT: Pharmacometrics & Systems. Pharmacol. 2021;10(5):399–411. https://doi.org/10.1002/psp4.12600.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Current Pharmacol Rep. 2016;2:161–9. https://doi.org/10.1007/s40495-016-0059-9.
Wilson CG, Aarons L, Augustijns P, Brouwers J, Darwich AS, De Waal T, Garbacz G, Hansmann S, Hoc D, Ivanova A, Koziolek M. Integration of advanced methods and models to study drug absorption and related processes: An UNGAP perspective. Eur J Pharm Sci. 2022;1(172):106100. https://doi.org/10.1016/j.ejps.2021.106100.
Frechen S, Rostami-Hodjegan A. Quality assurance of PBPK modeling platforms and guidance on building, evaluating, verifying and applying PBPK models prudently under the umbrella of qualification: why, when, what, how and by whom? Pharm Res. 2022;39(8):1733–48. https://doi.org/10.1007/s11095-022-03250-w.
Varma MV, Steyn SJ, Allerton C, El-Kattan AF. Predicting clearance mechanism in drug discovery: extended clearance classification system (ECCS). Pharm Res. 2015;32:3785–802. https://doi.org/10.1007/s11095-015-1749-4.
Guideline on quality of oral modified release products. Committee for Medicinal Products for Human Use (CHMP) 2012; Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-oral-modified-release-products_en.pdf. Accessed on 15 Dec 2022
Extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 1997. https://www.fda.gov/media/70939/download. Accessed on 15 Dec 2022
Guideline On the Investigation of Bioequivalence. Committee for Medicinal Products For Human Use (CHMP) 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed on 15 Dec 2022
Bioavailability and bioequivalence studies submitted in NDAs or INDs — general considerations. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) 2014. https://www.fda.gov/files/drugs/published/Bioavailability-and-Bioequivalence-Studies-Submitted-in-NDAs-or-INDs-%E2%80%94-General-Considerations.pdf. Accessed on 15 Dec 2022
Mitra A, Suarez-Sharp S, Pepin XJ, Flanagan T, Zhao Y, Kotzagiorgis E, Parrott N, Sharan S, Tistaert C, Heimbach T, Zolnik B. Applications of physiologically based biopharmaceutics modeling (PBBM) to support drug product quality: a workshop summary report. J Pharm Sci. 2021;110(2):594–609. https://doi.org/10.1016/j.xphs.2020.10.059.
Ahmad A, Pepin X, Aarons L, Wang Y, Darwich AS, Wood JM, Tannergren C, Karlsson E, Patterson C, Thörn H, Ruston L. IMI–Oral biopharmaceutics tools project–Evaluation of bottom-up PBPK prediction success part 4: Prediction accuracy and software comparisons with improved data and modelling strategies. Eur J Pharm Biopharm. 2020;1(156):50–63. https://doi.org/10.1016/j.ejpb.2020.08.006.
Jereb R, Kristl A, Mitra A. Prediction of fasted and fed bioequivalence for immediate release drug products using physiologically based biopharmaceutics modeling (PBBM). Eur J Pharm Sci. 2020;1(155):105554. https://doi.org/10.1016/j.ejps.2020.105554.
Yuvaneshwari K, Kollipara S, Ahmed T, Chachad S. Applications of PBPK/PBBM modeling in generic product development: an industry perspective. J Drug Deliv Sci Technol. 2022;2:103152. https://doi.org/10.1016/j.jddst.2022.103152.
Bhattiprolu AK, Kollipara S, Ahmed T, Boddu R, Chachad S. Utility of physiologically based biopharmaceutics modeling (PBBM) in regulatory perspective: application to supersede f2, enabling biowaivers & creation of dissolution safe space. J Pharm Sci. 2022;111(12):3397–410. https://doi.org/10.1016/j.xphs.2022.09.003.
Safety Reporting Requirements for INDs and BA/BE Studies. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) 2012; Available from: https://www.fda.gov/files/drugs/published/Safety-Reporting-Requirements-for-INDs-%28Investigational-New-Drug-Applications%29-and-BA-BE-%28Bioavailability-Bioequivalence%29-Studies.pdf. Accessed on 15 Dec 2022
SUPAC-IR: Immediate-release solid oral dosage forms: scale-up and post-approval changes: chemistry, manufacturing and controls, in vitro dissolution testing, and in vivo bioequivalence documentation. Center for Drug Evaluation and Research 1995; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ir-immediate-release-solid-oral-dosage-forms-scale-and-post-approval-changes-chemistry. Accessed on 15 Dec 2022
Loisios-Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. case example: Naproxen. Eur J Pharm Sci. 2020;15(143):105170. https://doi.org/10.1016/j.ejps.2019.105170.
Clinically relevant dissolution specifications: a biopharmaceutics’ risk based approach: an FDA perspective. USFDA 2021; Available from: https://www.apsgb.co.uk/wp-content/uploads/2021/05/Clinically-Relevant-Dissolution-Specifications-an-FDA-Perspective-__Om-Anand.pdf. Accessed on 15 Dec 2022
Kollipara S, Bhattiprolu AK, Boddu R, Ahmed T, Chachad S. Best practices for integration of dissolution data into physiologically based biopharmaceutics models (PBBM): a biopharmaceutics modeling scientist perspective. AAPS PharmSciTech. 2023;24(2):59. https://doi.org/10.1208/s12249-023-02521-y.
Sugano K. Lost in modelling and simulation? ADMET DMPK. 2021;9(2):75–109. https://doi.org/10.5599/admet.923.
Jereb R, Opara J, Legen I, Petek B, Grabnar-Peklar D. In vitro–in vivo relationship and bioequivalence prediction for modified-release capsules based on a PBPK absorption model. AAPS PharmSciTech. 2020;21:1–1. https://doi.org/10.1208/s12249-019-1566-x.2020.
Aishwarya R, Murthy A, Ahmed T, Chachad S. A novel approach to justify dissolution differences in an extended release drug product using physiologically based biopharmaceutics modeling and simulation. Journal of Pharmaceutical Sciences. 2022;111(6):1820–32. https://doi.org/10.1016/j.xphs.2022.02.007.
GastroPlus: mechanistic deconvolution and the future role of physiological modeling in IVIVC; Available from: https://pqri.org/wp-content/uploads/2015/08/pdf/Bolger.pdf. Accessed on 10th march 2023
Simcyp PBPK for drug-drug interactions (DDIs): a regulatory imperative; Available from: https://www.certara.com/app/uploads/2021/12/WP_DDI-v2.pdf. Accessed on 20th jan 2023
Wang W, Ouyang D. Opportunities and challenges of physiologically based pharmacokinetic modeling in drug delivery. Drug Dis Today. 2022. https://doi.org/10.1016/j.drudis.2022.04.015
Krstevska A, Đuriš J, Ibrić S, Cvijić S. In-depth analysis of physiologically based pharmacokinetic (PBPK) modeling utilization in different application fields using text mining tools. Pharm. 2022;15(1):107. https://doi.org/10.3390/pharmaceutics15010107.
Flanagan T, Van Peer A, Lindahl A. Use of physiologically relevant biopharmaceutics tools within the pharmaceutical industry and in regulatory sciences: where are we now and what are the gaps? Eur J Pharm Sci. 2016;25(91):84–90. https://doi.org/10.1016/j.ejps.2016.06.006.
Jamei M, Abrahamsson B, Brown J, Bevernage J, Bolger MB, Heimbach T, Karlsson E, Kotzagiorgis E, Lindahl A, McAllister M, Mullin JM. Current status and future opportunities for incorporation of dissolution data in PBPK modeling for pharmaceutical development and regulatory applications: OrBiTo consortium commentary. Eur J Pharm Biopharm. 2020;1(155):55–68. https://doi.org/10.1016/j.ejpb.2020.08.005.
Tang C, Ou-Yang CX, Chen WJ, Zou C, Huang J, Cui C, Yang S, Guo C, Yang XY, Lin Y, Pei Q. Prediction of pharmacokinetic parameters of inhaled indacaterol formulation in healthy volunteers using physiologically-based pharmacokinetic (PBPK) model. Eur J Pharm Sci. 2022;1(168):106055. https://doi.org/10.1016/j.ejps.2021.106055.
Mueller-Zsigmondy M, Limoncini FM. White paper-biopharmaceutics modelling as a fundamental tool to support accelerated access. efpia Website. 2020(a):n-an. https://oak.novartis.com/id/eprint/42879
Zhang T, Wells E. A review of current methods for food effect prediction during drug development. Curr Pharm Rep. 2020;6(5):267–79. https://doi.org/10.1007/s40495-020-00230-9.
Kambayashi A, Kiyota T, Fujiwara M, Dressman JB. PBPK modeling coupled with biorelevant dissolution to forecast the oral performance of amorphous solid dispersion formulations. Eur J Pharm Sci. 2019;1(135):83–90. https://doi.org/10.1016/j.ejps.2019.05.013.
Tannergren C, Jadhav H, Eckernäs E, Fagerberg J, Augustijns P, Sjögren E. Physiologically based biopharmaceutics modeling of regional and colon absorption in humans. Eur J Pharm Biopharm. 2023;1(186):144–59. https://doi.org/10.1016/j.ejpb.2023.03.013.
Stamatopoulos K, Ferrini P, Nguyen D, Zhang Y, Butler JM, Hall J, Mistry N. Integrating in vitro biopharmaceutics into physiologically based biopharmaceutic model (PBBM) to predict food effect of BCS IV zwitterionic drug (GSK3640254). Pharm. 2023;15(2):521. https://doi.org/10.3390/pharmaceutics15020521.
Lee JB, Zgair A, Taha DA, Zang X, Kagan L, Kim TH, Kim MG, Yun HY, Fischer PM, Gershkovich P. Quantitative analysis of lab-to-lab variability in Caco-2 permeability assays. Eur J Pharm Biopharm. 2017;1(114):38–42. https://doi.org/10.1016/j.ejpb.2016.12.027.
Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm Res. 2022;39(8):1669–80. https://doi.org/10.1007/s11095-022-03288-w.
Zhao L, Kim MJ, Zhang L, Lionberger R. Generating model integrated evidence for generic drug development and assessment. Clin Pharmacol Ther. 2019;105(2):338–49. https://doi.org/10.1002/cpt.1282.
Acknowledgements
Authors would like to thank Dr Reddy’s Laboratories Ltd. for providing an opportunity to publish this work.
Funding
This work was funded by Dr Reddy’s Laboratories Ltd. No other funding was received for this work.
Author information
Authors and Affiliations
Contributions
PK—concept and design, writing manuscript; MG—concept and design, writing manuscript; AM—concept and design, writing manuscript, manuscript review; and TA—concept and design, manuscript review, approval for version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
All the authors are employees of Dr Reddy’s Laboratories Ltd. and report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karnati, P., Murthy, A., Gundeti, M. et al. Modelling Based Approaches to Support Generic Drug Regulatory Submissions-Practical Considerations and Case Studies. AAPS J 25, 63 (2023). https://doi.org/10.1208/s12248-023-00831-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00831-4