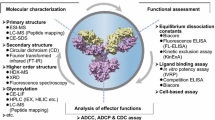
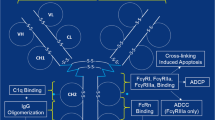
Abstract
Abstract. The development of monoclonal antibody (mAb) biosimilars is a complex process. The key to their successful development and commercialization is an in-depth understanding of the key product attributes that impact safety and efficacy and the strategies to control them. Functional assessment of mAb is a crucial part of the comparability of biopharmaceutical drugs. The development of a relevant and robust functional assay requires an interdisciplinary approach and sufficient flexibility to balance regulatory concerns as well as dynamics and variability during the manufacturing process. Although many advanced tools are available to study and compare the potency and bioactivity of the protein, most of these techniques suffer from major shortcomings that limit their routine use. These include the complexity of the task, establishment of the relevance of the chosen method with the mechanism of action (MOA) of the biosimilar, cost and extended time of analysis, and often the ambiguity in interpretation of the resulting data. To overcome or to address these challenges, the use of multiple orthogonal state-of-the-art techniques is a necessary prerequisite.
Graphical abstract



Similar content being viewed by others
References
Rathore AS, Chirmule N, Malani H. Reimagining affordable biosimilars. BioPharm Int. 2020;33(10):16–22.
Rathore AS, Bhargava A. Biosimilars in developed economies: overview, status, and regulatory considerations. Regul Toxicol Pharmacol. Academic Press. 2020;110:104525. https://doi.org/10.1016/j.yrtph.2019.104525.
Tsuruta LR. Lopes dos Santos M, Moro AM. Biosimilars advancements: moving on to the future. Biotechnol Prog. 2015;31(5):1139–49. https://doi.org/10.1002/btpr.2066.
Frapaise FX. The end of phase 3 clinical trials in biosimilars development? BioDrugs. 2018;32(4):319–24. https://doi.org/10.1007/s40259-018-0287-0.
Berkowitz SA, Engen JR, Mazzeo JR, Jones GB. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012;11(7):527–40. https://doi.org/10.1038/nrd3746.
Láng JA, Balogh ZC, Nyitrai MF, Juhász C, Gilicze AKB, Iliás A, et al. In vitro functional characterization of biosimilar therapeutic antibodies. Drug Discov Today Technol. 2020. https://doi.org/10.1016/j.ddtec.2020.11.010.
Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. Elsevier Current Trends. 2019;37(1):9–16. https://doi.org/10.1016/j.tibtech.2018.05.014.
Biosimilars approved in the US (Available from: https://www.gabionline.net/biosimilars/general/Biosimilars-approved-in-the-US ) [cited 2021 Oct 11].
Biosimilars approved in Europe (Available from: https://gabionline.net/biosimilars/general/biosimilars-approved-in-europe) [cited 2021 Oct 11].
Moorkens E, Vulto AG, Huys I. An overview of patents on therapeutic monoclonal antibodies in Europe: are they a hurdle to biosimilar market entry? MAbs. 2020;12(1):1743517. https://doi.org/10.1080/19420862.2020.1743517.
Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12(1):1703531. https://doi.org/10.1080/19420862.2019.1703531.
Kaplon H, Reichert JM. Antibodies to watch in 2021. MAbs. 2021;13(1):1860476. https://doi.org/10.1080/19420862.2020.1860476.
Arosio P, Rima S, Morbidelli M. Aggregation mechanism of an IgG2 and two IgG1 monoclonal antibodies at low pH: from oligomers to larger aggregates. Pharm Res. 2013;30(3):641–54. https://doi.org/10.1007/s11095-012-0885-3.
Steplewski Z, Sun LK, Shearman CW, Ghrayeb J, Daddona P, Koprowski H. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc Natl Acad Sci U S A. 1988;85(13):4852–6. https://doi.org/10.1073/pnas.85.13.4852.
Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianférani S. Characterization of therapeutic antibodies and related products. Anal Chem. 2013;85(2):715–36. https://doi.org/10.1021/ac3032355.
Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335–45. https://doi.org/10.1007/s10456-004-8272-2.
Paek K, Kim GW, Ahn SY, Lim JH, Jung D, Kim S, et al. Assessment of the molecular mechanism of action of SB3, a Trastuzumab Biosimilar. BioDrugs. 2019;33(6):661–71. https://doi.org/10.1007/s40259-019-00381-2.
Yang D, Kroe-Barrett R, Singh S, Roberts CJ, Laue TM. IgG cooperativity - is there allostery? Implications for antibody functions and therapeutic antibody development. MAbs. 2017;9(8):1231–52. https://doi.org/10.1080/19420862.2017.1367074.
Beyer B, Schuster M, Jungbauer A, Lingg N. Microheterogeneity of recombinant antibodies: analytics and functional impact. Biotechnol J. 2018;13(1). https://doi.org/10.1002/biot.201700476.
Rosales C. Fcγ receptor heterogeneity in leukocyte functional responses. Front Immunol. 2017;8:280. https://doi.org/10.3389/fimmu.2017.00280.
T, Sosa K, Casaco A, López-Requena A, Mateo de Acosta C. Expression and biological characterization of an anti-CD20 biosimilar candidate antibody: a case study. MAbs. 2012;4(4):488–96. https://doi.org/10.4161/mabs.20761.
Bielsky MC, Cook A, Wallington A, Exley A, Kauser S, Hay JL, et al. Streamlined approval of biosimilars: moving on from the confirmatory efficacy trial. Drug Discov Today Elsevier Current Trends. 2020;25(11):1910–8. https://doi.org/10.1016/j.drudis.2020.09.006.
da Silva A, Kronthaler U, Koppenburg V, Fink M, Meyer I, Papandrikopoulou A, et al. Target-directed development and preclinical characterization of the proposed biosimilar rituximab GP2013. Leuk Lymphoma. 2014;55(7):1609–17. https://doi.org/10.3109/10428194.2013.843090.
Lee KH, Lee J, Bae JS, Kim YJ, Kang HA, Kim SH, et al. Analytical similarity assessment of rituximab biosimilar CT-P10 to reference medicinal product. MAbs. 2018;10(3):380–96. https://doi.org/10.1080/19420862.2018.1433976.
Urbano PC, Soccol VT, Azevedo VF. Apoptosis and the FLIP and NF-kappa B proteins as pharmacodynamic criteria for biosimilar TNF-alpha antagonists. Biologics. 2014;8:211–20. https://doi.org/10.2147/BTT.S57253.
White JR, Abodeely M, Ahmed S, Debauve G, Johnson E, Meyer DM, et al. Best practices in bioassay development to support registration of biopharmaceuticals. Biotechniques. 2019;67(3):126–37. https://doi.org/10.2144/btn-2019-0031.
Lu Y, Vernes JM, Chiang N, Ou Q, Ding J, Adams C, et al. Identification of IgG(1) variants with increased affinity to FcγRIIIa and unaltered affinity to FcγRI and FcRn: comparison of soluble receptor-based and cell-based binding assays. J Immunol Methods. 2011;365(1-2):132–41. https://doi.org/10.1016/j.jim.2010.12.014.
Pollard TD. A guide to simple and informative binding assays. Mol Biol Cell. 2010;21(23):4061–7. https://doi.org/10.1091/mbc.E10-08-0683.
Wild D. The immunoassay handbook: theory and applications of ligand binding, ELISA and related techniques. 4th ed: Elsevier; 2013.
Lebakken CS, Riddle SM, Singh U, Frazee WJ, Eliason HC, Gao Y, et al. Development and applications of a broad-coverage, TR-FRET-based kinase binding assay platform. J Biomol Screen. 2009;14(8):924–35. https://doi.org/10.1177/1087057109339207.
Noto A, Ngauv P, Trautmann L. Cell-based flow cytometry assay to measure cytotoxic activity. J Vis Exp. 2013;82:e51105. https://doi.org/10.3791/51105.
Cooper MA. Label-free screening of bio-molecular interactions. Anal Bioanal Chem. 2003;377:834–42. https://doi.org/10.1007/s00216-003-2111-y.
Schasfoort RBM. Handbook of surface plasmon resonance. 2nd ed: RSC Publishing; 2017.
Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, et al. Label-free detection of biomolecular interactions using BioLayer interferometry for kinetic characterization. Comb Chem High Throughput Screen. 2009;12(8):791–800. https://doi.org/10.2174/138620709789104915.
Estep P, Reid F, Nauman C, Liu Y, Sun T, Sun J, et al. High throughput solution-based measurement of antibody-antigen affinity and epitope binning. MAbs. 2013;5(2):270–8. https://doi.org/10.4161/mabs.23049.
DiLillo DJ, Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res. 2015;3(7):704–13. https://doi.org/10.1158/2326-6066.
Datta-Mannan A, Wroblewski VJ. Application of FcRn binding assays to guide mAb development. Drug Metab Dispos. 2014;42(11):1867–72. https://doi.org/10.1124/dmd.114.059089.
Hintersteiner B, Lingg N, Zhang P, Woen S, Hoi KM, Stranner S, et al. Charge heterogeneity: Basic antibody charge variants with increased binding to Fc receptors. MAbs. 2016;8(8):1548–60. https://doi.org/10.1080/19420862.2016.1225642.
Ishino T, Wang M, Mosyak L, Tam A, Duan W, Svenson K, et al. Engineering a monomeric Fc domain modality by N-glycosylation for the half-life extension of biotherapeutics. J Biol Chem. 2013;288(23):16529–37. https://doi.org/10.1074/jbc.M113.457689.
Branstetter E, Duff RJ, Kuhns S, Padaki R. Fc glycan sialylation of biotherapeutic monoclonal antibodies has limited impact on antibody-dependent cellular cytotoxicity. FEBS Open Bio. 2021;11(11):2943–9. https://doi.org/10.1002/2211-5463.13267.
Zhang Y, Mathur A, Maher G, Arroll T, Bailey R. Impact of IgG2 high molecular weight species on neonatal Fc receptor binding assays. Anal Biochem. 2015;489:25–31. https://doi.org/10.1016/j.ab.2015.07.017.
Bielefeld-Sevigny M. AlphaLISA immunoassay platform- the “no-wash” high-throughput alternative to ELISA. Assay Drug Dev Technol. 2009;7(1):90–2. https://doi.org/10.1089/adt.2009.9996.
Prasad A, Lautenschlager C. A Comparison of AlphaLISA and TR-FRET homogeneous immunoassays in serum-containing samples. Application Note 2009.
Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, et al. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278(47):46974–82. https://doi.org/10.1074/jbc.M307764200.
Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164(8):4178–84. https://doi.org/10.4049/jimmunol.164.8.4178.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. https://doi.org/10.3389/fimmu.2014.00520.
Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104(20):8433–7. https://doi.org/10.1073/pnas.0702936104.
Patel R, Neill A, Liu H, Andrien B. IgG subclass specificity to C1q determined by surface plasmon resonance using Protein L capture technique. Anal Biochem. 2015;479:15–7. https://doi.org/10.1016/j.ab.2015.03.012.
Zhou W, Lin S, Chen R, Liu J, Li Y. Characterization of antibody-C1q interactions by Biolayer Interferometry. Anal Biochem. 2018;549:143–8. https://doi.org/10.1016/j.ab.2018.03.022.
Liu C, Morrow KJ. Biosimilars of monoclonal antibodies: a practical guide to manufacturing preclinical, and clinical development. Wiley Online. Library. 2016.
Velasco-Velázquez MA, Salinas-Jazmín N, Hisaki-Itaya E, Cobos-Puc L, Xolalpa W, González G, et al. Extensive preclinical evaluation of an infliximab biosimilar candidate. Eur J Pharm Sci. 2017;102:35–45. https://doi.org/10.1016/j.ejps.2017.01.038.
Hu J, Wala I, Han H, Nagatani J, Barger T, Civoli F, et al. Comparison of cell-based and non-cell-based assay platforms for the detection of clinically relevant anti-drug neutralizing antibodies for immunogenicity assessment of therapeutic proteins. J Immunol Methods. 2015;419:1–8. https://doi.org/10.1016/j.jim.2015.02.006.
Eon-Duval A, Broly H, Gleixner R. Quality attributes of recombinant therapeutic proteins: an assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol Prog. 2012;28(3):608–22. https://doi.org/10.1002/btpr.1548.
Rathore AS. Follow-on protein products: scientific issues, developments and challenges. Trends Biotechnol. 2009;27(12):698–705. https://doi.org/10.1016/j.tibtech.2009.09.004.
Han YS, Lee JE, Jung JW, Lee JS. Inhibitory effects of bevacizumab on angiogenesis and corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):541–8. https://doi.org/10.1007/s00417-008-0976-3.
Bala K, Ambwani K, Gohil NK. Effect of different mitogens and serum concentration on HUVEC morphology and characteristics: implication on use of higher passage cells. Tissue Cell. 2011;43(4):216–22. https://doi.org/10.1016/j.tice.2011.03.004.
Afify SM, Sanchez Calle A, Hassan G, Kumon K, Nawara HM, Zahra MH, et al. A novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br J Cancer. 2020;122(9):1378–90. https://doi.org/10.1038/s41416-020-0792-z.
Lundholt BK, Scudder KM, Pagliaro L. A simple technique for reducing edge effect in cell-based assays. J Biomol Screen. 2003;8(5):566–70. https://doi.org/10.1177/1087057103256465.
Govindarajulu Z. Statistical techniques in bioassay. 2nd ed: Karger Publishers; 2001.
Gottschalk PG, Dunn JR. Measuring parallelism, linearity, and relative potency in bioassay and immunoassay data. J Biopharm Stat. 2005;15(3):437–63. https://doi.org/10.1081/BIP-200056532.
Malo N, Hanley JA, Cerquozzi S, Pelletier J, Nadon R. Statistical practice in high-throughput screening data analysis. Nat Biotechnol. 2006;24(2):167–75. https://doi.org/10.1038/nbt1186.
Little TA. Essentials in bioassay design and relative potency determination. Biopharm Int. 2016;29(4):49–52.
Fedorov VV, Leonov SL. Optimal design of dose response experiments: a model-oriented approach. Drug Inf J. 2001;35(4):1373–83. https://doi.org/10.1177/009286150103500433.
Little AT. Out-of-trend identification and removal in stability modelling and regression analysis. Biopharm Int. 2016;29(1):50–5.
USP_1033_Biological Assay Validation. 2010 The United States Pharmacopeial Convention.
Seo N, Polozova A, Zhang M, Yates Z, Cao S, Li H, et al. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. MAbs. 2018;10(4):678–91. https://doi.org/10.1080/19420862.2018.1452580.
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, et al. Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2(6):613–24. https://doi.org/10.4161/mabs.2.6.13333.
Tanetsugu Y, Tagami T, Terukina T, Ogawa T, Ohta M, Ozeki T. Development of a sustainable release system for a ranibizumab biosimilar using poly(lactic-co-glycolic acid) biodegradable polymer-based microparticles as a platform. Biol Pharm Bull. 2017;40(2):145–50. https://doi.org/10.1248/bpb.b16-00437.
Méry B, Guy JB, Vallard A, Espenel S, Ardail D, Rodriguez-Lafrasse C, et al. In vitro cell death determination for drug discovery: a landscape review of real issues. J Cell Death. 2017;10:1179670717691251. https://doi.org/10.1177/1179670717691251.
Brennan FR, Kiessling A. In vitro assays supporting the safety assessment of immunomodulatory monoclonal antibodies. Toxicol in Vitro. 2017;45(Pt 3):296–308. https://doi.org/10.1016/j.tiv.2017.02.025.
Shealy DJ, Cai A, Staquet K, Baker A, Lacy ER, Johns L, et al. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. MAbs. 2010;2(4):428–39. https://doi.org/10.4161/mabs.12304.
Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16.
Suresh T, Lee LX, Joshi J, Barta SK. New antibody approaches to lymphoma therapy. J Hematol Oncol. 2014;7:58. https://doi.org/10.1186/s13045-014-0058-4.
Majewska NI, Tejada ML, Betenbaugh MJ, Agarwal N. N-Glycosylation of IgG and IgG-like recombinant therapeutic proteins: why is it important and how can we control it? Annu Rev Chem Biomol Eng. 2020;11:311–38. https://doi.org/10.1146/annurev-chembioeng-102419-010001.
Kute T, Stehle JR Jr, Ornelles D, Walker N, Delbono O, Vaughn JP. Understanding key assay parameters that affect measurements of trastuzumab-mediated ADCC against Her2 positive breast cancer cells. Oncoimmunology. 2012;1(6):810–21. https://doi.org/10.4161/onci.20447.
Schnueriger A, Grau R, Sondermann P, Schreitmueller T, Marti S, Zocher M. Development of a quantitative, cell-line based assay to measure ADCC activity mediated by therapeutic antibodies. Mol Immunol. 2011;48(12-13):1512–7. https://doi.org/10.1016/j.molimm.2011.04.010.
Cheng ZJ, Garvin D, Paguio A, Moravec R, Engel L, Fan F, et al. Development of a robust reporter-based ADCC assay with frozen, thaw-and-use cells to measure Fc effector function of therapeutic antibodies. J Immunol Methods. 2014;414:69–81. https://doi.org/10.1016/j.jim.2014.07.010.
Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs. 2012;4(3):326–40. https://doi.org/10.4161/mabs.19941.
Nupur N, Chhabra N, Dash R, Rathore AS. Assessment of structural and functional similarity of biosimilar products: rituximab as a case study. MAbs. 2018;10(1):143–58. https://doi.org/10.1080/19420862.2017.1402996.
Visser J, Feuerstein I, Stangler T, Schmiederer T, Fritsch C, Schiestl M. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs. 2013;27(5):495–507. https://doi.org/10.1007/s40259-013-0036-3.
Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. https://doi.org/10.1155/2011/379123.
Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711. https://doi.org/10.1080/19420862.2018.1466767.
Herter S, Herting F, Muth G, van Puijenbroek E, Schlothauer T, Ferrara C, et al. GA101 P329GLALA, a variant of obinutuzumab with abolished ADCC, ADCP and CDC function but retained cell death induction, is as efficient as rituximab in B-cell depletion and antitumor activity. Haematologica. 2018;103(2):e78–81. https://doi.org/10.3324/haematol.2017.178996.
Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11:307. https://doi.org/10.1186/1479-5876-11-307.
Rahalkar H, Cetintas HC, Salek S. Quality, Non-clinical and clinical considerations for biosimilar monoclonal antibody development: EU, WHO, USA, Canada, and BRICS-TM regulatory guidelines. Front Pharmacol. 2018;9:1079. https://doi.org/10.3389/fphar.2018.01079.
Kang J, Kim SY, Vallejo D, Hageman TS, White DR, Benet A, et al. Multifaceted assessment of rituximab biosimilarity: the impact of glycan microheterogeneity on Fc function. Eur J Pharm Biopharm. 2020;146:111–24. https://doi.org/10.1016/j.ejpb.2019.12.003.
Xu Y, Xie L, Zhang E, Gao W, Wang L, Cao Y, et al. Physicochemical and functional assessments demonstrating analytical similarity between rituximab biosimilar HLX01 and the MabThera®. MAbs. 2019;11(3):606–20. https://doi.org/10.1080/19420862.2019.1578147.
Cerutti ML, Pesce A, Bès C, Seigelchifer M. Physicochemical and biological characterization of RTXM83, a new rituximab biosimilar. BioDrugs. 2019;33(3):307–19. https://doi.org/10.1007/s40259-019-00349-2.
Montacir O, Montacir H, Eravci M, Springer A, Hinderlich S, Saadati A, et al. Comparability study of Rituximab originator and follow-on biopharmaceutical. J Pharm Biomed Anal. 2017;140:239–51. https://doi.org/10.1016/j.jpba.2017.03.029.
Cuello HA, Segatori VI, Alberto M, Pesce A, Alonso DF, Gabri MR. Comparability of antibody-mediated cell killing activity between a proposed biosimilar RTXM83 and the originator rituximab. BioDrugs. 2016;30(3):225–31. https://doi.org/10.1007/s40259-016-0171-8.
Azevedo V, Dela Coletta Troiano Araujo L, Bassalobre Galli N, Kleinfelder A, Marostica Catolino N, Martins Urbano PC. Adalimumab: a review of the reference product and biosimilars. Biosimilars. 2016;6:29–44. https://doi.org/10.2147/BS.S98177.
Huizinga TWJ, Torii Y, Muniz R. Adalimumab biosimilars in the treatment of rheumatoid arthritis: a systematic review of the evidence for biosimilarity. Rheumatol Ther. 2021;8(1):41–61. https://doi.org/10.1007/s40744-020-00259-8.
Argollo M, Fiorino G, Gilardi D, Furfaro F, Roda G, Loy L, et al. Biosimilars of adalimumab in inflammatory bowel disease: are we ready for that? Curr Pharm Des. 2019;25(1):7–12. https://doi.org/10.2174/1381612825666190312113610.
Hillson J, Mant T, Rosano M, Huntenburg C, Alai-Safar M, Darne S, et al. Pharmacokinetic equivalence, comparable safety, and immunogenicity of an adalimumab biosimilar product (M923) to Humira in healthy subjects. Pharmacol Res Perspect. 2018;6(1):e00380. https://doi.org/10.1002/prp2.380.
Magnenat L, Palmese A, Frémaux C, D'amici F, Terlizzese M, Rossi, et al. Demonstration of physicochemical and functional similarity between the proposed biosimilar adalimumab MSB11022 and Humira®. mAbs. 2017;9:127–39. https://doi.org/10.1080/19420862.2016.1259046.
Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology. 2015;25(12):1325–34. https://doi.org/10.1093/glycob/cwv065.
Mimura Y, Katoh T, Saldova R, O'Flaherty R, Izumi T, Mimura-Kimura Y, et al. Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy. Protein Cell. 2018;9(1):47–62. https://doi.org/10.1007/s13238-017-0433-3.
Pace D, Lewis N, Wu T, Gillespie R, Leiske D, Velayudhan J, et al. Characterizing the effect of multiple Fc glycan attributes on the effector functions and FcγRIIIa receptor binding activity of an IgG1 antibody. Biotechnol Prog. 2016;32(5):1181–92. https://doi.org/10.1002/btpr.2300.
Wen Y, Jawa V. The impact of product and process related critical quality attributes on immunogenicity and adverse immunological effects of biotherapeutics. J Pharm Sci. 2021;110(3):1025–41. https://doi.org/10.1016/j.xphs.2020.12.003.
Bansal R, Dash R, Rathore AS. Impact of mAb aggregation on its biological activity: rituximab as a case study. J Pharm Sci. 2020;109(9):2684–98. https://doi.org/10.1016/j.xphs.2020.05.015.
Dash R, Rathore AS. Freeze thaw and lyophilization induced alteration in mAb therapeutics: trastuzumab as a case study. J Pharm Biomed Anal. 2021;201:114122. https://doi.org/10.1016/j.jpba.2021.114122.
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, Yao Z, Sreedhara A, Cano T, Tesar D, Nijem I, Allison DE, Wong PY, Kao YH, Quan C, et al. Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2(6):613–24. https://doi.org/10.4161/mabs.2.6.13333.
Hajba L, Szekrényes Á, Borza B, Guttman A. On the glycosylation aspects of biosimilarity. Drug Discov Today. 2018;23(3):616–25. https://doi.org/10.1016/j.drudis.2018.01.009.
Duivelshof BL, Jiskoot W, Beck A, Veuthey J-L, Guillarme D, D’Atri V. Glycosylation of biosimilars: recent advances in analytical characterization and clinical implications. Anal Chim Acta. 2019;1089:1–18. https://doi.org/10.1016/j.aca.2019.08.044.
Kang J, Pisupati K, Benet A, Ruotolo BT, Schwendeman SP, Schwendeman A. Infliximab biosimilars in the age of personalized medicine. Trends Biotechnol. 2018;36(10):987–92. https://doi.org/10.1016/j.tibtech.2018.05.002.
Pisupati K, Tian Y, Okbazghi S, Benet A, Ackermann R, Ford M, et al. A multidimensional analytical comparison of remicade and the biosimilar remsima. Anal Chem. 2017;89(9):4838–46. https://doi.org/10.1021/acs.analchem.6b04436.
Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology. 2015;25(12):1325–34. https://doi.org/10.1093/gly-cob/cwv065.
Acknowledgements
This work was funded by the Centre of Excellence for Biopharmaceutical Technology grant from the Department of Biotechnology, Ministry of Science and Technology (BT/COE/34/SP15097/2015). RD acknowledges the Indian Council of Medical Research (5/3/8/56/ITR-F/2018-ITR), New Delhi, India, for her fellowship.
Author information
Authors and Affiliations
Contributions
All authors planned the review. RD and SKS wrote the first draft of the paper. NC and ASR supervised the project. ASR was responsible for the funding and reviewed and edited the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Website: www.biotechCMZ.com
Rights and permissions
About this article
Cite this article
Dash, R., Singh, S.K., Chirmule, N. et al. Assessment of Functional Characterization and Comparability of Biotherapeutics: a Review. AAPS J 24, 15 (2022). https://doi.org/10.1208/s12248-021-00671-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00671-0