Abstract
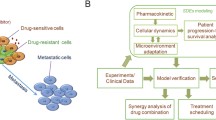
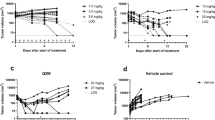
The self-renewal and differentiation of cancer stem-like cells (CSCs) leads to cellular heterogeneity, causing one of the greatest challenges in cancer therapy. Growing evidence suggests that CSC-targeting therapy enhances the effect of concomitant antitumour therapy. To gain an in-depth understanding of this enhanced effect, the kinetic profile of estimated CSC frequency (the fraction of CSCs in tumour) was evaluated for in vivo characterization of cellular heterogeneity using sunitinib and dopamine as a paradigm combination therapy. Female MCF-7/Adr xenografted Balb/c nude mice were treated with sunitinib (p.o., 20 mg/kg) and dopamine (i.p., 50 mg/kg), alone or in combination. Estimated CSC frequency and tumour size were measured over time. Mechanistic PK/PD modelling was performed to quantitatively describe the relationship between drug concentration, estimated CSC frequency and tumour size. Sunitinib reduced tumour size by inducing apoptosis of differentiated tumour cells (DTCs) and enriched CSCs by stimulating its proliferation. Dopamine exhibited anti-CSC effects by suppressing the capacity of CSCs and inducing its differentiation. Simulation and animal studies indicated that concurrent administration was superior to sequential administration under current experimental conditions. Alongside tumour size, the current study provides mechanistic insights into the estimation of CSC frequency as an indicator for cellular heterogeneity. This forms the conceptual basis for in vivo characterization of other combination therapies in preclinical cancer studies.







Similar content being viewed by others
References
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–37.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44.
Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45(8):872–7.
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9.
Brooks MD, Burness ML, Wicha MS. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell. 2015;17(3):260–71.
Ablett MP, Singh JK, Clarke RB. Stem cells in breast tumours: are they ready for the clinic? Eur J Cancer. 2012;48(14):2104–16.
Wang S, Mou Z, Ma Y, Li J, Li J, Ji X, et al. Dopamine enhances the response of sunitinib in the treatment of drug-resistant breast cancer: involvement of eradicating cancer stem-like cells. Biochem Pharmacol. 2015;95(2):98–109.
Hao F, Wang S, Zhu X, Xue J, Li J, Wang L, et al. Pharmacokinetic-pharmacodynamic modeling of the anti-tumor effect of sunitinib combined with dopamine in the human non-small cell lung Cancer Xenograft. Pharm Res. 2017;34(2):408–18.
Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18 Suppl 10(suppl_10):x3–10.
Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356(2):323–8.
Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 2012;109(8):2784–9.
Chinchar E, Makey K, Gibson J, Chen F, Cole S, Megason G, et al. Sunitinib significantly suppresses the proliferation, migration, apoptosis resistance, tumor angiogenesis and growth of triple-negative breast cancers but increases breast cancer stem cells. Vasc Cell. 2014;6(1):1–12.
Mackey JR, Kerbel RS, Gelmon KA, McLeod DM, Chia SK, Rayson D, et al. Controlling angiogenesis in breast cancer: a systematic review of anti-angiogenic trials. Cancer Treat Rev. 2012;38(6):673–88.
Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–97.
Ribba B, Holford NH, Magni P, Trocóniz I, Gueorguieva I, Girard P, et al. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacometrics Syst Pharmacol. 2014;3(5):1–10.
Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64(11):937–46.
Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM User’s Guides. Ellicott City: Icon Development Solutions; 2009. p. 2009.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2(6):e50.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160(1–2):62–73.
Li J, Li J, Wang S, Yuan Y, Su Q, Lu W, et al. Simultaneous determination of sunitinib and its active metabolites N-desethyl sunitinib (SU12662) in nude mice plasma by liquid chromatography tandem mass spectrometry and its application to a pharmacokinetic study. J Chin Pharm Sci. 2015;24(4):217–24.
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35.
Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210.
Ganguly R, Puri IK. Mathematical model for the cancer stem cell hypothesis. Cell Prolif. 2006;39(1):3–14.
Koch G, Walz A, Lahu G, Schropp J. Modeling of tumor growth and anticancer effects of combination therapy. J Pharmacokinet Pharmacodyn. 2009;36(2):179–97.
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, et al. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res. 2004;64(3):1094–101.
Al Faraj A, Shaik AS, Al Sayed B, Halwani R, Al JI. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality nanoprobes. Nanomed (London, England). 2016;11(1):31–46.
Ganguly R, Puri IK. Mathematical model for chemotherapeutic drug efficacy in arresting tumour growth based on the cancer stem cell hypothesis. Cell Prolif. 2007;40(3):338–54.
Molina-Pena R, Alvarez MM. A simple mathematical model based on the cancer stem cell hypothesis suggests kinetic commonalities in solid tumor growth. PLoS One. 2012;7(2):e26233.
Ma YH, Wang SY, Ren YP, Li J, Guo TJ, Lu W, et al. Antitumor effect of axitinib combined with dopamine and PK-PD modeling in the treatment of human breast cancer xenograft. Acta Pharmacol Sin. 2018;40(2):243–56.
Karlsson MO, Jonsson EN, Wiltse CG, Wade JR. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26(2):207–46.
Workgroup EM, Marshall SF, Burghaus R, Cosson V, Cheung SY, Chenel M, et al. Good practices in model-informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst Pharmacol. 2016;5(3):93–122.
Ooi Q-X, Wright DF, Isbister GK, Duffull SB. Evaluation of assumptions underpinning pharmacometric models. AAPS J. 2019;21(5):97.
Noori S, Friedlich P, Seri I. Pharmacology review developmentally regulated cardiovascular, renal, and neuroendocrine effects of dopamine. NeoReviews. 2003;4(10):e283–e8.
Felip E, Massuti B, Camps C, Benito D, Isla D, Gonzalez-Larriba JL, et al. Superiority of sequential versus concurrent administration of paclitaxel with etoposide in advanced non-small cell lung cancer: comparison of two phase II trials. Clin Cancer Res. 1998;4(11):2723–8.
Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29(34):4491–7.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84.
Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14(8):2502–10.
Zhang L, Yao HJ, Yu Y, Zhang Y, Li RJ, Ju RJ, et al. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33(2):565–82.
Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28(25):4006–12.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–91.
Acknowledgements
The authors thank Prof. Stephen Duffull (Otago Pharmacometrics Group, University of Otago) for his careful reading of the manuscript.
Funding
This study is sponsored by National Natural Science Foundation of China (NSFC) [Grant 81473277 and 81703605].
Author information
Authors and Affiliations
Contributions
S.W. performed experiments and wrote the paper. X.Z analysed the data and wrote the paper. M. H. and F.H. performed experiments. W.L. designed experiments. T.Z. designed experiments and wrote the paper.
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 505 kb)
Appendices
Appendices
Existing Knowledge
Here, we summarise the existing knowledge of CSC theory and the related pharmacological activities of dopamine and sunitinib. All information was incorporated into a mechanistic model that described the sunitinib/dopamine combination in the treatment of drug-resistant breast cancer:
- 1)
The cancer stem-like cells (CSCs) and the differentiated tumour cells (DTCs) own the capacity of converting to the other one [1].
- 2)
The size of CSCs is much smaller than DTCs [38].
- 3)
The DTC population grow more aggressively and mainly contribute to the tumour bulk [39].
- 4)
The CSC population cycle very slowly and eventually reach the steady state during the evolution of tumour [23, 40].
- 5)
Dopamine can eradicate CSCs [7].
- 6)
Sunitinib is a multi-targeted receptor tyrosine kinase inhibitor (TKI) with anti-angiogenic and antitumour activities [9, 10].
- 7)
Sunitinib enriches the CSC population within the tumour [11,12,13].
Derivation of the Equation for Tumour Volume
In this study, the tumour volume is approximated by the sum of volumes of different cancel cells. As the tumour mainly consists of two representative cell subpopulations (i.e. CSCs and DTCs), the tumour volumes at time 0 (Eq. A1) and t (Eq. A2) are derived:
Here, V0 and V(t) represent the tumour volume at time 0 and t. CSC(t) and DTC(t) denote the amount of CSCs or DTCs at time t, respectively. Vs − CSC and Vs − DTC represent the volume of a single cell of CSC or DTC, respectively.
Re-organizing the Eqs. A1 and A2 yields the expression of tumour volume at time t related to the tumour volume at time 0 (Eq. A3):
Dividing the numerator and denominator by Vs − DTC yields Eq. A4:
Here, Ratio represents the volume ratio of a single CSC to a single DTC.
Since the exact amount of initial tumour cells is unknown, the initial amount of total tumour cells is assumed to be 100 (arbitrary units). From our experiments, the average estimated CSC frequency before the first drug administration is 1.55%. Thus, the initial amount of CSCs (CSC0) is 1.55 units, and the initial amount of DTCs (DTC0) is 98.45 units. In addition, it is known that the size of CSCs is much smaller than DTCs (Existing knowledge 2), indicating that the volume ratio of a single CSC to a single DTC (Ratio) is much smaller than 1. Therefore, the product of CSC0 and Ratio (CSC0 ∙ Ratio) is much smaller than DTC0. Thus, Eq. A4 can be reduced into Eq. A5.
It is known that the DTC population grow more aggressively and mainly contribute to the tumour bulk (Existing knowledge 3), indicating that DTC(t) is much larger than CSC(t). Furthermore, Ratio is much smaller than 1 (Existing knowledge 2). Hence, the product of CSC(t) and Ratio is much smaller than DTC(t). Thus, Eq. A5 can be reduced into the final expression of tumour volume (Eq. A6):
Rights and permissions
About this article
Cite this article
Wang, S., Zhu, X., Han, M. et al. Mechanistic Pharmacokinetic/Pharmacodynamic Model of Sunitinib and Dopamine in MCF-7/Adr Xenografts: Linking Cellular Heterogeneity to Tumour Burden. AAPS J 22, 45 (2020). https://doi.org/10.1208/s12248-020-0428-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-0428-5