Abstract
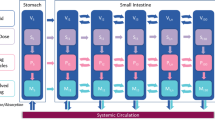
Gastrointestinal (GI) fluid volume and its dynamic change are integral to study drug disintegration, dissolution, transit, and absorption. However, key questions regarding the local volume and its absorption, secretion, and transit remain unanswered. The dynamic fluid compartment absorption and transit (DFCAT) model is proposed to estimate in vivo GI volume and GI fluid transport based on magnetic resonance imaging (MRI) quantified fluid volume. The model was validated using GI local concentration of phenol red in human GI tract, which was directly measured by human GI intubation study after oral dosing of non-absorbable phenol red. The measured local GI concentration of phenol red ranged from 0.05 to 168 μg/mL (stomach), to 563 μg/mL (duodenum), to 202 μg/mL (proximal jejunum), and to 478 μg/mL (distal jejunum). The DFCAT model characterized observed MRI fluid volume and its dynamic changes from 275 to 46.5 mL in stomach (from 0 to 30 min) with mucus layer volume of 40 mL. The volumes of the 30 small intestine compartments were characterized by a max of 14.98 mL to a min of 0.26 mL (0–120 min) and a mucus layer volume of 5 mL per compartment. Regional fluid volumes over 0 to 120 min ranged from 5.6 to 20.38 mL in the proximal small intestine, 36.4 to 44.08 mL in distal small intestine, and from 42 to 64.46 mL in total small intestine. The DFCAT model can be applied to predict drug dissolution and absorption in the human GI tract with future improvements.







Similar content being viewed by others
References
Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11(2):217–24.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21.
Sjogren E, Thorn H, Tannergren C. In Silico modeling of gastrointestinal drug absorption: predictive performance of three physiologically based absorption models. Mol Pharm. 2016;13(6):1763–78.
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186(2):119–25.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41–67.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–37.
Hellström PM, Grybäck P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol. 2006;20(3):397–407.
Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11(9):3039–47.
Greger R, Windhorst U. Comprehensive Human Physiology: From Cellular Mechanisms to Integration. Berlin: Springer; 2013.
Roy H. Short textbook of surgery. New Delhi: Jaypee Brothers Medical Publishers Pvt. Limited; 2010. p. 202.
Johansson MEV, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2013.
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20.
Derrien M, van Passel MWJ, van de Bovenkamp JHB, Schipper RG, de Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1(4):254–68.
Ferrua MJ, Singh RP. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J Food Sci. 2010;75(7):R151–R62.
Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int J Pharm. 1996;140(1):111–8.
Péronnet F, Mignault D, du Souich P, Vergne S, Le Bellego L, Jimenez L, et al. Pharmacokinetic analysis of absorption, distribution and disappearance of ingested water labeled with D(2)O in humans. Eur J Appl Physiol. 2012;112(6):2213–22.
Ogungbenro K, Pertinez H, Aarons L. Empirical and semi-mechanistic Modelling of double-peaked pharmacokinetic profile phenomenon due to gastric emptying. AAPS J. 2015;17(1):227–36.
Davenport HW. Physiology of the digestive tract: an introductory text, vol. vii. Chicago: Year Book Medical Publishers; 1982. p. 245.
Hounnou G, Destrieux C, Desmé J, Bertrand P, Velut S. Anatomical study of the length of the human intestine. Surg Radiol Anat. 2002;24(5):290–4.
Cronin CG, Delappe E, Lohan DG, Roche C, Murphy JM. Normal small bowel wall characteristics on MR enterography. Eur J Radiol. 2010;75(2):207–11.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19(3):359–76.
Lennernäs H, Aarons L, Augustijns P, Beato S, Bolger M, Box K, et al. Oral biopharmaceutics tools – time for a new initiative – an introduction to the IMI project OrBiTo. Eur J Pharm Sci. 2014;57:292–9.
Hoad CL, Marciani L, Foley S, Totman JJ, Wright J, Bush D, et al. Non-invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol. 2007;52(23):6909–22.
Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2005;22(10):971–9.
Shingaki T, Takashima T, Wada Y, Tanaka M, Kataoka M, Ishii A, et al. Imaging of gastrointestinal absorption and biodistribution of an orally administered probe using positron emission tomography in humans. Clin Pharmacol Ther. 2012;91(4):653–9.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
The study was approved by University of Michigan IRBMED HUM00085066 and the Food and Drug Administration (RIHSC protocol 14-029D. Study volunteers provided written informed consent. The study was in accordance with study protocol, the International Conference on Harmonization of Good Clinical Practice guidelines, and applicable local regulatory requirements. The ClinicalTrials.gov identifier is NCT02806869.
Additional information
Guest Editors: Peng Zou, Doanh Tran, and Edward Bashaw
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yu, A., Jackson, T., Tsume, Y. et al. Mechanistic Fluid Transport Model to Estimate Gastrointestinal Fluid Volume and Its Dynamic Change Over Time. AAPS J 19, 1682–1690 (2017). https://doi.org/10.1208/s12248-017-0145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0145-x