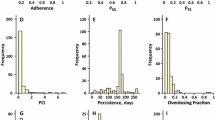
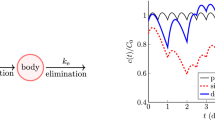
Abstract
Partial adherence with a prescribed or randomly assigned dose gives rise to unintended variability in actual drug exposure in clinical practice and during clinical trials. There are tremendous costs associated with incomplete and/or improper drug intake—to both individual patients and society as a whole. Methodology for quantifying the relation between adherence, exposure and drug response is an area of active research. Modeling and statistical approaches have been useful in evaluating the impact of adherence on therapeutics and in addressing the challenges of confounding and measurement error which arise in this context. This paper reviews quantitative approaches to using adherence information in improving therapeutics. It draws heavily on applications in the area of HIV pharmacology.
Similar content being viewed by others
References
Vrijens B, Tousset E, Gaillard P, Metry J, Urquhart J. Major features of dose omissions in 87 ambulatory drug trials.Clin Pharmacol Ther. 2005;77:P99.
Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance.Am J Hosp Pharm. 1991;48:1978–1988.
Cramer JA, Spilker B, eds. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press Ltd; 1991.
Didlake RH, Dreyfus K, Kermanet RH, et al. Patient noncompliance: a major cause of late graft failure in cyclosporine-treated renal transplants.Transplant Proc. 1988;20(suppl 3):63–69.
Kastrissios H, Blaschke TF. Therapeutic implications of nonadherence with antiretroviral drug regimens.HIV. 1998;8:24–28.
Urquhart J. Ascertaining how much compliance is enough with outpatient antibiotic regimens.Postgrad Med J. 1992;68(suppl 3):S49-S59.
Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy.Clin Pharmacokinet. 1997;32:345–356.
Hong JH, Sumrani N, Delaney V, et al. Causes of late renal allograft failure in the ciclosporin era.Nephron. 1992;62:272–279.
Dunn J, Golden D, Van Buren CT, et al. Causes of graft loss beyond two years in the cyclosporine era.Transplantation. 1990;49:349–353.
Urquhart J. Role of patient compliance in clinical pharmacokinetics. A review of recent research.Clin Pharmacokinet. 1994;27:202–215.
Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care.J Clin Epidemiol. 1997;50:385–391.
Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique.JAMA. 1989;261:3273–3277.
Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world. Effectiveness versus efficacy studies.Pharmacoeconomics. 1999;15:423–434.
Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis.N Engl J Med. 1994;330:1179–1184.
Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice.Clin Ther. 1999;21:1074–1090.
Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG).AIDS Care. 2000;12: 255–266.
de Klerk E, van der Linden SJ. Compliance monitoring of NSAID drug therapy in ankylosing spondylitis, experiences with an electronic monitoring device.Br J Rheumatol. 1996;35:60–65.
Lee JY, Kusek JW, Greene PF, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study.Am J Hypertens. 1996;9:719–725.
Bloch M, Gur E, Shalev AY. Hypouricemic effect of zuclopenthixol: a potential marker of drug compliance?Psychopharmacology (Berl). 1992;109:377–378.
Kapur S, Ganguli R, Ulrich R, et al. Use of random-sequence riboflavin as a marker of medication compliance in chronic schizophrenics.Schizophr Res. 1991;6:49–53.
Hardy E, Kumar S, Peaker S, et al. A comparison of a short half-life marker (low-dose isoniazid), a long half-life pharmacological indicator (low-dose phenobarbitone) and measurements of a controlled release ‘therapeutic drug’ (metoprolol, Metoros) in reflecting incomplete compliance by volunteers.Br J Clin Pharmacol. 1990;30:437–441.
Maenpaa H, Javela K, Pikkarainen JH, et al. Minimal doses of digoxin: a new marker for compliance to medication.Eur Heart J. 1987;8(suppl 1):31–37.
Pekovic V, Mayanja H, Vjecha M, et al. Comparison of three composite compliance indices in a trial of self-administered preventive therapy for tuberculosis in HIV-infected Ugandan adults. Uganda-Case Western Reserve University Research Collaboration.J Clin Epidemiol. 1998;51:597–607.
Lu J, Gries JM, Verotta D, et al. Selecting reliable pharmacokinetic data for explanatory analyses of clinical trials in the presence of possible noncompliance.J Pharmacokinet Pharmacodyn. 2001;28:343–362.
Melbourne KM, Geletko SM, Brown SL, et al. Medication adherence in patients with HIV infection: a comparison of two measurement methods.AIDS Read. 1999;9:329–338.
Carroll RJ, Ruppert D, Stefanski LA. Measurement error in nonlinear models. Boca Raton, FL: CRC Press, 1998.
Kenna LA, Sheiner LB. Estimating treatment effect in the presence of non-compliance measured with error: precision and robustness of data analysis methods.Stat Med. 2004;23:3561–3580.
Kenna LA. Estimating treatment effect in the presence of non-compliance measured with error: power, precision and robustness of data analysis methods [dissertation].PhD Thesis. San Francisco, CA; University of California San Francisco: 2001.
Catania JA, Binson D, Canchola J, et al. Effects of interviewer gender, interviewer choice, and item wording on response to questions concerning sexual behavior.Public Opinion Quarterly. 1996;60:345–375.
Kaplan RM, Simon HJ. Compliance in medical care: reconsideration of self-predictions.Ann Behav Med. 1990;12:66–71.
Hyland ME, Kenyon CA, Allen R, et al. Diary keeping in asthma: comparison of written and electronic methods.BMJ. 1993;306:487–489.
Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population.AIDS. 2000;14:357–366.
Feinstein AR. On white-coat effects and the electronic monitoring of compliance.Arch Intern Med. 1990;150:1377–1378.
Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors.Ann Intern Med. 2001;134:968–977.
Vrijens B, Goetghebeur E. Comparing compliance patterns between randomized treatments.Control Clin Trials. 1997;18:187–203.
Robins JM. Correction for non-compliance in equivalence trials.Stat Med. 1998;17:269–302; discussion 387–389.
Goetghebeur E, Molenberghs G, Katz J. Estimating the causal effect of compliance on binary outcome in randomized controlled trials.Stat Med. 1998;17:341–355.
Efron B, Feldman D. Compliance as an explanatory variable in clinical trials.J Am Stat Assoc. 1991;86:9–22.
Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials.Clin Pharmacol Ther. 1995;57:6–15.
Angrist JD, Imbens GW, Rubin DR. Identification of causal effects using instrumental variables.J Am Stat Assoc. 1996;91:444–472.
Albert JM, Demets DL. On a model-based approach to estimating efficacy in clinical trials.Stat Med. 1994;13:2323–2335.
Jonsson EN, Wade JR, Almqvist G, et al. Discrimination between rival dosing histories.Pharm Res. 1997;14:984–991.
Cheng CW, Woo KS, Chan JC, et al. Association between adherence to statin therapy and lipid control in Hong Kong Chinese patients at high risk of coronary heart disease.Br J Clin Pharmacol. 2004;58:528–535.
Frolkis JP, Pearce GL, Nambi V, et al. Statins do not meet expectations for lowering low-density lipoprotein cholesterol levels when used in clinical practice.Am J Med. 2002;113:625–629.
Simons LA, Levis G, Simons J. Apparent discontinuation rates in patients prescribed lipid-lowering drugs.Med J Aust. 1996;164:208–211.
Hiatt JG, Shamsie SG, Schectman G. Discontinuation rates of cholesterol-lowering medications: implications for primary care.Am J Manag Care. 1999;5:437–444.
Larsen J, Andersen M, Kragstrup J, et al. High persistence of statin use in a Danish population: compliance study 1993–1998.Br J Clin Pharmacol. 2002;53:375–378.
Hughes DA, Walley T. Predicting “real world” effectiveness by integrating adherence with pharmacodynamic modeling.Clin Pharmacol Ther. 2003;74:1–8.
Williams-Deane M, Potter LS. Current oral contraceptive use instructions: An analysis of patient package inserts.Fam Plann Perspect. 1992;24:111–115.
Urquhar J. Erratic patient compliance with prescribed drug regiments: target for drug delivery systems.Clin Pharmacol Ther. 2000;67:331–334.
Cramer JA. Effect of partial compliance on cardiovascular medication effectiveness.Heart. 2002;88:203–206.
Johnson BF, Whelton A. A study design for comparing the effects of missing daily doses of antihypertensive drugs.Am J Ther. 1994;1:260–267.
Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol.Br J Clin Pharmacol. 1982;13(suppl 2):345S-351S.
Vaur L, Dutrey-Dupagne C, Boussac J, et al. Differential effects of a missed dose of trandolapril and enalapril on blood pressure control in hypertensive patients.J Cardiovasc Pharmacol. 1995;26:127–131.
Leenen FH, Fourney A, Notman G, et al. Persistence of antihypertensive effect after ‘missed doses’ of calcium antagonist with long (amlodipine) vs short (diltiazem), elimination half-life.Br J Clin Pharmacol. 1996;41:83–88.
Mallion JM, Meilhac B, Tremel F, et al. Use of a microprocessor-equipped tablet box in monitoring compliance with antihypertensive treatment.J Cardiovasc Pharmacol. 1992;19(suppl 2):S41-S48.
Lee SY, Song XY. Bayesian analysis of structural equation models with dichotomous variables.Stat Med. 2003;22:3073–3088.
Wainberg MA, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance.JAMA. 1998;279:1997–1983.
Vanhove GF, Schapiro JM, Winters MA, et al. Patient compliance and drug failure in protease inhibitor monotherapy.JAMA. 1996;276:1955–1956.
Hirsch MS, Conway B, D'Aquila RT, et al. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. International AIDS Society-USA Panel.JAMA. 1998;279:1984–1991.
Bangsberg DR, Charlebois ED, Grant RM, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations.AIDS. 2003;17:1925–1932.
Pfister M, Labbe L, Hammer SM, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398.Antimicrob Agents Chemother. 2003;47:130–137.
Urquhart J. Non-compliance: the ultimate absorption barrier. In: Prescott LF, Nimmo WS, eds. Novel Drug Delivery and Its Therapeutic Applications, Chichester, UK: John Wiley & Sons, 1989;127–137.
Kastrissios H, Suarez JR, Katzenstein D, et al. Characterizing patterns of drug-taking behavior with a multiple drug regimen in an AIDS clinical trial.AIDS. 1998;12:2295–2303.
Girard P, Sheiner LB, Kastrissios H, et al. Do we need full compliance data for population pharmacokinetic analysis?J Pharmacokinet Biopharm. 1996;24:265–282.
Girard P, Blaschke TF, Kastrissios H, et al. A Markov mixed effect regression model for drug compliance.Stat Med. 1998;17:2313–2333.
Vrijens B. Analyzing time-varying patterns of human exposure to xenobiotics and their biomedical impact. Ghent, Belgium: University of Ghent; 2002.
Labbé L, Hammer SM, Mellors JW, et al. A Markov model for the effect of covariates including drug adherence on longitudinal viral response in HIV patients.Clin Pharmacol Ther. 2004;75:P90.
Labbé L, Verotta D. A non-linear mixed effect dynamic model using adherence to treatment to describe long-term therapy outcome in HIV-patients.Clin Pharmacol Ther. 2005;77:P90.
Diggle P, Kenward MG. Informative drop-out in longitudinal data analysis.Appl Stats. 1994;43:49–93.
Hu C, Sale ME. A joint model for nonlinear longitudinal data with informative dropout.J Pharmacokinet Pharmacodyn. 2003;30:83–103.
Noordmohamed SE, Henry WK, Rhame FS, et al. Strategies for control of zidovudine concentrations in serum.Antimicrob Agents Chemother. 1995;39:2792–2797.
Goldie SJ, Paltiel AD, Weinstein MC, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection.Am J Med. 2003;115:632–641.
Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions.Ann Intern Med. 1999;131:81–87.
Glazer WM, Ereshefsky L. A pharmacoeconomic model of outpatient antipsychotic therapy in “revolving door” schizophrenic patients.J Clin Psychiatry. 1996;57:337–345.
Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits.Arch Intern Med. 1990;150:1509–1510.
Waeber B, Leonetti G, Kolloch R, et al. Compliance with aspirin or placebo in the Hypertension Optimal Treatment (HOT) study.J Hypertens. 1999;17:1041–1045.
Kastrissios H, Blaschke TF. Medication compliance as a feature in drug development.Annu Rev Pharmacol Toxicol. 1997;37:451–475.
Kass MA, Meltzer DW, Gordon M, et al. Compliance with topical pilocarpine treatment.Am J Ophthalmol. 1986;101:515–523.
Peck CC. Non-compliance and clinical trials: regulatory perspectives. In: Metry JM, Meyer UA, eds. Drug Regimen Compliance: Issues in Clinical Trials and Patient Management. Chichester, UK: John Wiley & Sons, 1999;97–102.
Hasford J. Design and analysis of clinical trials of compliance, In: Metry JM, Meyer UA, eds. Drug Regimen Compliance: Issues in Clinical Trials and Patient Management. Chichester, UK: John Wiley & Sons, 1999;23–40.
Haynes RB, Montague P, Oliver T, et al. Interventions for helping patients to follow prescriptions for medications.Cochrane Database Syst Rev. 2000;2:CD000011.
Volmink J, Garner P. Directly observed therapy for treating tuberculosis.Cochrane Database Syst Rev. 2001;4:CD003343.
Barker J, Millard J. Directly observed therapy and treatment adherence.Lancet. 2000;356:1030–1; author reply 1032.
Urquhart J, Chevalley C. Impact of unrecognized dosing errors on the cost and effectiveness of pharmaceuticals.Drug Info J. 1998;22:363–378.
Sheiner LB. Learning versus confirming in clinical drug development.Clin Pharmacol Ther. 1997;61:275–291.
Girard P. Clinical trial simulation: a tool for understanding study failures and preventing them.Basic Clin Pharmacol Toxicol. 2005;96:228–234.
Lasagn L, Hutt PB. Health care, research, and regulatory impact of noncompliance. In: Cramer JA, Spilker B, eds. Compliance in Medical Practice and Clinical Trials, JA. New York: Raven Press, 1991;393–403.
Morris SE, Groom GV, Cameron ED, et al. Studies on low dose oral contraceptives: plasma hormone changes in relation to deliberate pill (‘Microgynon 30’) omission.Contraception. 1979;20:61–69.
Chowdhury V, Joshi UM, Gopalkrishna K, et al. ‘Escape’ ovulation in women due to the missing of low dose combination oral contraceptive pills.Contraception. 1980;22:241–247.
Wang E, Shi S, Cekan SZ, et al. Hormonal consequences of “missing the pill”.Contraception. 1982;26: 545–566.
Landgren BM, Diczfalusy E. Hormonal consequences of missing the pill during the first two days of three consecutive artificial cycles.Contraception. 1984;29:437–446.
Landgren BM, Csemiczky G. The effect of follicular growth and luteal function of “missing the pill”. A comparison between a monophasic and a triphasic combined oral contraceptive.Contraception. 1991;43:149–159.
Cross JT, Lee HD, Nelson JS, et al. One in five marketed drugs undergoes a dosage change: 1980–1999.Clin Pharmacol Ther. 2001;69:P63.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies.JAMA. 1998;279:1200–1205.
Fremont-Smith K, Kravitz GR, Bush T, et al. Adverse drug reactions in hospitalized patients.J Am Stat Assoc. 1998;280:1741.
Burney KD, Krishnan K, Ruffin MT, et al. Adherence to single daily dose of aspirin in a chemoprevention trial. An evaluation of self-report and microelectronic monitoring.Arch Farm Med. 1996;5:297–300.
Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications.Ann Intern Med. 2001;134:1004–1006.
Meredith PA, Elliott HL. Therapeutic coverage: reducing the risks of partial compliance.Br J Clin Pract. 1994;73(suppl):13–17.
Detry JM. Patient compliance and therapeutic coverage: amlodipine versus nifedipine SR in the treatment of hypertension and angina: interim results. Steering Committee and Cardiologists and General Practitioners involved in the Belgium Multicentre Study on Patient Compliance.Clin Cardiol. 1994;17(suppl 3):III12-III16.
Urquhart J, De Klerk E. Contending paradigms for the interpretation of data on patient compliance with therapeutic drug regimens.Stat Med. 1998;17:251–267; discussion 387–389.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: October 5, 2005
Rights and permissions
About this article
Cite this article
Kenna, L.A., Labbé, L., Barrett, J.S. et al. Modeling and simulation of adherence: Approaches and applications in therapeutics. AAPS J 7, 40 (2005). https://doi.org/10.1208/aapsj070240
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj070240