Abstract
Background
Lumbar plexus block (LPB) is a proven modality to provide analgesia following lower limb surgeries. The present study compared the effect of buprenorphine in different doses viz. 150 μg and 300 μg as an adjuvant to levobupivacaine in unilateral lumbar plexus block. In this prospective, controlled, and double-blind study, ninety patients undergoing hip, thigh, and knee surgeries under subarachnoid block were enrolled. The patients were randomly allocated into three groups of thirty each, to receive LPB with 0.25% levobupivacaine plain (group L), 0.25% levobupivacaine with 150 μg buprenorphine (group BL), or 0.25% levobupivacaine with 300 μg buprenorphine (group BH), after the sensory level of subarachnoid receded to T10. Total volume administered was 30 ml. The duration of analgesia post LPB, total pain-free period, cumulative rescue analgesic doses per patient, number of patients requiring rescue analgesic, pain scores using visual analog scale (VAS), and sedation levels were noted at protocolized predetermined intervals in each case.
Results
The duration of analgesia post LPB was significantly prolonged in both the buprenorphine groups (9.76 ± 1.39 h in group with 150 μg buprenorphine and 10.13 ± 1.5 h in group with 300 μg buprenorphine) as compared to 4.25 ± 0.93 h in the control group (p < 0.001). The total pain free-period of 12.81 ± 1.49 h was maximum in group BH as compared to 12.42 ± 1.47 h in group BL and 7.01 ± 0.89 h in group L and was statistically significant with the control group (p = 0.001). The cumulative rescue analgesic doses per patient was also significantly higher in control group L (3.10 ± 0.40) as compared to groups BL (1.77 ± 0.5) and BH (1.33 ± 0.48). There was significant decrease in pain scores in patients of both buprenorphine groups compared to the control group up to 24 h (p < 0.001). In group BH, patients were sedated in the first hour with a modified Ramsay Sedation Score of 1.93 ± 0.86 which was statistically significant from the group L (modified RSS of 1.00 ± 0.00, p = 0.003) as well as from group BL (modified RSS of 1.47 ± 0.50, p = 0.043).
Conclusions
Buprenorphine in either of the doses (150 μg or 300 μg) as an adjuvant to levobupivacaine in lumbar plexus block provided comparable postoperative analgesia. A dose of 300 μg, however, resulted in significant sedation and respiratory depression. Hence, buprenorphine 150 μg appears to be an optimal dose providing prolonged postoperative analgesia and minimal sedation.
Similar content being viewed by others
Background
The lumbar plexus block (LPB) provides anesthesia or analgesia to the entire distribution of the lumbar plexus, including the anterolateral and medial thigh, the knee, and to the saphenous nerve below the knee (Stevens et al. 2000). The last decade of clinical practice has witnessed a revolution in how regional anesthesia is performed using the ultrasound. Ultrasound imaging allows direct visualization of peripheral nerves, the block needle tip, and local anesthetic distribution. Levobupivacaine, the pure (S)-enantiomer of bupivacaine, has less cardiac and central nervous system toxicity compared to bupivacaine (Bardsley et al. 1998). Its duration of sensory block is longer than ropivacaine, so it has the advantage in providing prolonged postoperative pain control (Gonzalez-Suarez et al. 2009). Buprenorphine, a μ-receptor partial agonist opioid, is similar in structure to morphine but approximately 33 times more potent when compared to it. Buprenorphine has been used for effective pain control via various routes (Fukuda 2015). However, there is paucity in literature regarding its efficacy when added to levobupivacaine for lumbar plexus block.
This study was designed to compare the analgesic efficacy of buprenorphine in different doses (viz. 150 μg and 300 μg) as an adjuvant to levobupivacaine in unilateral LPB in patients undergoing surgeries around the hip, thigh, and knees under subarachnoid block.
Methods
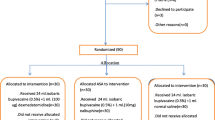
After institutional ethics committee approval and registration with Clinical Trials Registry - India with CTRI No. CTRI/2018/01/011437, ninety patients of American Society of Anesthesiologists (ASA) physical status 1 or 2, aged between 18 and 60 years, scheduled for hip, thigh, and knee surgeries under subarachnoid block were included in this prospective, randomized, and double-blind study. The procedure of lumbar plexus block was explained to the patient to obtain informed written consent. Patients who refused regional anesthesia, having an infection at the block site or allergy to study drugs, coagulation disorders, peripheral neuropathies, or opioid-tolerant or opioid-dependent patients were excluded from the study.
The enrolled patients were randomized into 3 groups by computer-generated random number chart. The random number was enclosed in a sealed opaque envelope opened by an investigator to know the drug combination to be administered.
In group L, patients received 15 ml of 0.5% levobupivacaine + 15 ml normal saline. Patients in group BL received 15 ml of 0.5% levobupivacaine + 150 μg (0.5 ml) buprenorphine + 14.5 ml normal saline. In group BH, patients were administered 15 ml of 0.5% levobupivacaine + 300 μg (1 ml) buprenorphine + 14 ml normal saline. The final concentration of levobupivacaine used was 0.25%, and the total volume of drug solution was 30 ml in all the groups.
Preoperatively, all patients were instructed regarding how to read the VAS that was used for assessing the pain in the postoperative period. All patients were kept nil orally for 8 h before the procedure and were given premedication in the form of tablet alprazolam 0.25 mg and tablet ranitidine 150 mg the night before and at 6:00 AM on the day of surgery, with a sip of water. Patients were given SAB under standard monitoring using 3.0 cc (15 mg) of 0.5% hyperbaric bupivacaine, and surgery was commenced after confirming adequate effect of SAB.
After completion of surgery, the patients were shifted to post anesthesia care unit (PACU) and monitored for the regression of sensory block to T10 level, following which they were given LPB with 30 ml of the prepared drug solution, using ultrasound guidance under strict aseptic precautions by a trained anesthesiologist, who was blinded to the prepared drug solution. All the procedures performed were in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Subsequently, each patient was observed for pain, vitals, and side effects at hourly intervals as mentioned in the proforma (i.e., 0, 1, 2, 4, 6, 12, 18, and 24 h) for 24 h by an anesthesiologist blinded to group assignment. For the first 24 h, the protocol for postoperative pain consisted of standard orders of i.v. diclofenac 1.5 mg/kg stat for VAS > 4 and, for breakthrough pain, i.v. tramadol hydrochloride 2 mg/kg as and when required. The patients were evaluated for the following in the postoperative unit-
-
◦ Duration of analgesia post LPB—measured from the time after giving LPB to the administration of 1st rescue analgesic
-
◦ Total pain free interval—measured from the time after giving SAB to the administration of 1st rescue analgesic
-
◦ The cumulative rescue analgesic doses per patient in each group and number of patients requiring rescue analgesia at different time intervals up to 24 h
-
◦ Pain scores using 11-point VAS, where 0 is no pain and 10 is worst imaginable pain
-
◦ Hemodynamic parameter variability in heart rate, systolic blood pressure, diastolic blood pressure, and mean blood pressure
-
◦ Side effects—patients were asked to rate the severity of nausea and vomiting on a 4-point scale (1—none, 4—severe); sedation was evaluated using modified Ramsay Sedation Score (RSS)
The primary outcome of the study was the duration of analgesia post LPB. The secondary outcomes were the total pain free interval, pain scores (VAS), the cumulative rescue analgesic doses per patient in each group, number of patients needing rescue analgesia, and sedation levels at various time intervals.
Sample size was calculated based on a previous study by Jain et al. (2017), which computed that approximately 25 patients should be included in each group to detect clinically significant difference in duration of block and postoperative analgesia between the groups with alpha error of 0.05 with 90% power and 95% confidence limit. Assuming a 5% drop out rate, the final sample size was determined to retain sixty patients for better validation of results. Hence, each group had 30 patients in the present study.
The data was entered in MS Excel spreadsheet, and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0. Quantitative variables were compared using one-way ANOVA test between the three groups. To determine if the relationship between two sets of data was statistically significant, Tukey’s HSD (honestly significant difference) post hoc test was used.
Results
A total of 94 patients were recruited, out of which 4 patients were excluded from analysis due to block failure. All the 3 groups were comparable with respect to demographic variables such as age, gender, BMI, ASA physical status, and duration of surgery (Table 1).
In the present study, total duration of analgesia post LPB was significantly prolonged in both the buprenorphine groups (9.76 ± 1.39 h in group with 150 μg buprenorphine and 10.13 ± 1.5 h in group with 300 μg buprenorphine) as compared to 4.25 ± 0.93 h in the control group (p < 0.001). However, the duration of analgesia between both the buprenorphine groups (i.e., groups BL and BH) was statistically insignificant (p = 0.509) (Fig. 1).
The total pain-free period of 12.81 ± 1.49 h was maximum in group BH as compared to 12.42 ± 1.47 h in group BL and 7.01 ± 0.89 h in group L. The total pain free-period was statistically significant between the control group and both buprenorphine groups (p = 0.001); however, it was statistically insignificant between groups BL and BH (p = 0.480) (Table 2).
The cumulative rescue analgesic doses per patient in each group were calculated (Fig. 2). It was higher in group L (3.10 ± 0.40 doses) as compared to group BL (1.77 ± 0.50 doses) and group BH (1.33 ± 0.47doses) which was a statistically significant (p = 0.001) (Table 3). Hence, rescue analgesic requirement per patient in the control group (group L) was maximum followed by low dose (150 μg) buprenorphine group (group BL) and minimum in group BH (300 μg).
The number of patients requiring rescue analgesics at studied time interval was also calculated. It was statistically significant at 4 h (p < 0.001), 6 h (p < 0.001), 18 h (p = 0.027), and 24 h (p = < 0.001) (Table 4).
It was further observed that group L required rescue analgesics from the 4th hour onwards up to 24 h. Sixteen patients in this group required 1st rescue analgesic at 4th hour and the remaining 14 patients demanded 1st rescue analgesic at 6th hour. Patients in both the buprenorphine groups (groups BL and BH) required 1st rescue analgesia starting at 12th hour onwards, thereby indicating longer pain free-period with LPB containing buprenorphine as an adjuvant.
The mean VAS at 0, 1, 2, 4, 6, 12, 18, and 24 h in all the 3 groups was calculated. There was significant decrease in pain scores in patients of both buprenorphine groups (BL and BH) as compared to the control group up to 24 h (p < 0.001). The mean pain scores at all time frames except at 1 and 6 h were significantly higher in group L as compared to groups BL and BH (Fig. 3).
All patients were comfortable and responded to commands in postoperative period in groups L and BL. In group BH, patients were sedated in the first hour after LPB administration with a modified Ramsay Sedation Score of 1.93 ± 0.86. This was statistically significant from the group L (modified RSS of 1.00 ± 0.00, p = 0.003) as well as from group BL (modified RSS of 1.47 ± 0.50, p = 0.043). The maximum modified Ramsay Sedation Score reached was 4 in 2 patients, while 4 patients had achieved a RSS of 3 at 1 h post LPB administration in group BH, while the maximum sedation score reached in group BL was 2 and there was no sedation in the control group at any point of time post LPB administration. These patients required active awakening and supplemental oxygen to prevent any hypoxemia associated adverse events. There was no residual sedation in postoperative period after 2 h of performing block in either of the 3 groups (Fig. 4).
The oxygen saturation was normal and comparable between all the three groups in the rest of the study period (Fig. 5).
Further, 3 patients had nausea and vomiting in group BH, which was controlled with inj. ondansetron 4 mg i.v. stat. Apart from this, none of the patients in any group had any adverse effect at any point of time.
All the patients were hemodynamically stable in the postoperative period in all the three groups. Though there was a statistically significant increase in heart rate in control group (group L) as compared to the groups with buprenorphine (i.e., groups BL and BH) at 4 h, 12 h, 18 h, and 24 h after surgery, it was clinically insignificant and no intervention was required (Fig. 6).
The heart rate was comparable between the groups in the rest of the study period. There was a statistically significant difference in mean blood pressure in between group L and groups BL and BH at 4 h and 12 h and in between group BH and groups L and BL at 18 and 24 h, but it did not require any intervention (Fig. 7). The MBP was comparable between the groups in the rest of the study period.
Discussion
The results of the present study demonstrated that the addition of either 150 μg or 300 μg buprenorphine to 30 ml levobupivacaine 0.25% in unilateral lumbar plexus block provides prolonged duration of analgesia post LPB, longer pain free interval, decreased pain (VAS) score, and reduced analgesic requirement. However, the higher dose of 300 μg leads to significant sedation in first 2 h of the LPB.
The lumbar plexus block provides a valuable modality for postoperative analgesia as it covers the entire distribution of the lumbar plexus, including the anterolateral and medial thigh, the knee, and to the saphenous nerve below the knee, and the use of ultrasound has made the procedure precise and safe (Lumbar plexus block 2012). Studies conducted to determine the efficacy of LPB as a method of postoperative analgesia after hip surgeries under general anesthesia concluded that LPB provides effective analgesia, reducing postoperative analgesic requirements (Stevens et al. 2000; Sherif et al. 2011).
A study conducted by Sauter et al. to determine the minimum effective volume of local anesthetic and the effective doses for a sensory LPB, not requiring motor block of the femoral nerve, found that for a sensory lumbar plexus block, the ED95 was 25.8 ml (Sauter et al. 2015). Therefore, we have used 30 ml of 0.25% levobupivacaine in our study to provide a successful lumbar plexus block in almost all the patients under study.
Opioids are increasingly being used as an adjuvant in various regional blocks for their efficacy in prolonging the duration of block. It has been studied that perineurally administered opioids exert their analgesic effect through both central and peripheral mechanisms (Candido et al. 2002; Laduron 1984). The mechanism for centrally mediated analgesia has been postulated to be its easy penetration through the axonal myelin and nerve membrane and then centripetal axonal transport of opioids into substantia gelatinosa (Panditrao et al. 2011; Meunier 1997; Lutfy and Cowan 2004).
Buprenorphine interacts with the opioid receptor-like (ORL1) receptor, which has distinct pharmacological characteristics, activation of which leads to inhibition of the enzyme adenylate cyclase, calcium channel conductance, and activation of inwardly rectifying potassium channels (Connor et al. 1996a, b; Knoflach et al. 1996; Vaughan and Christie 1996). The analgesic effect of buprenorphine is the effect of the suppression of spinal synaptic transmission by alteration of these two ion channels (Leffler et al. 2012; Wajima et al. 1995). The intense action of buprenorphine peripherally is a potent property of buprenorphine as a local anesthetic is by binding to intramembranous part of receptor; it mediates the tonic and concentration dependent blockade of Na+ channels, thus blocking action potential in the terminal endings of C fibers. This property is greater than other opioids and several local anesthetics (Gormley et al. 1996; Takahashi et al. 2013).
A number of studies have been performed using buprenorphine as an adjuvant in various peripheral nerve blocks with encouraging results (Jain et al. 2017; Jadon et al. 2009; Behr et al. 2012; Paliwal and Karnawat 2013). Studies on the use of buprenorphine in lumbar plexus block were, however, lacking. Hence, we intended to study the effect of buprenorphine as an adjuvant to levobupivacaine in ultrasound-guided lumbar plexus block for postoperative analgesia in patients undergoing surgeries around the hip, thigh, and knee, under the subarachnoid block, simultaneously also comparing two different doses of buprenorphine viz. 150 μg and 300 μg to determine the optimal dose.
Murdoch et al. (2002) conducted a study comparing 3 concentrations of levobupivacaine viz. 0.0625%, 0.125%, and 0.25% for postoperative analgesia via continuous epidural infusion in 105 patients undergoing hip or knee replacement surgery. They concluded that levobupivacaine as a continuous epidural infusion provided adequate postoperative analgesia and that the 0.25% concentration provided significantly longer analgesia than 0.125% or 0.0625% levobupivacaine without any significant increase in detectable motor blockade relative to the 0.125% group; hence, a concentration of 0.25% was used in our study.
In the current study, the mean duration of analgesia post LPB was significantly prolonged in both the buprenorphine groups (9.76 ± 1.39 h in group BL and 10.13 ± 1.5 h in group BH) as compared to 4.25 ± 0.93 h in the control group (p = 0.000). Jain et al. (2017) used 300 μg buprenorphine as an adjuvant to 0.5% ropivacaine in the supraclavicular brachial plexus block. They reported an increase in the duration of analgesia of approximately 7 h as compared to the control group. This result of their study is consistent with our study, in which demand for 1st rescue analgesic was prolonged by approximately 6 h in 300 μg buprenorphine group. However, there was an overall increase in the duration of analgesia to up to 14.5 h in the buprenorphine group and up to 7.5 h in the control group in the study conducted by Jain et al. This may be due to the higher concentration of local anesthetic used (0.5% ropivacaine) in their study.
Similarly, Paliwal and Karnawat (2013) used 300 μg buprenorphine as an adjuvant with racemic bupivacaine 0.25% in supraclavicular brachial plexus block and found that the demand for 1st rescue analgesia was prolonged by approximately 7.5 h in the buprenorphine group. This is also in concordance with the present study. However, the total duration of analgesia was more in their study (6.16 ± 1.86 h in the control group and 13.71 ± 6.96 h in the buprenorphine group), which may be attributable to the higher volume of local anesthetic used by them in their study (40 ml in supraclavicular block, as compared to 30 ml in LPB in the present study).
The cumulative recue analgesic doses per patient and the total number of rescue analgesics doses in each group was significantly less in groups in which buprenorphine was administered (i.e., groups BL and BH), as compared to control group (group L) in our study. The control group required rescue analgesic doses from 4th hour onwards up to 24 h. Sixteen patients in group L required rescue analgesic at 4th hour and the remaining 14 patients demanded rescue analgesic at 6th hour. Patients in both the buprenorphine groups (groups BL and BH) required rescue analgesia starting at 12th hour, thereby indicating longer pain free-period with LPB containing buprenorphine as an adjuvant. These observations are also consistent with studies by Behr et al. (2012) and Paliwal and Karnawat (2013) (using either 150 μg or 300 μg of buprenorphine), where the authors have stated that the requirement of rescue analgesics was less when compared with the control group.
Our study shows significantly lower VAS scores with the use of buprenorphine in either dose (150 μg or 300 μg). A statistically significant difference was found in the VAS scores between groups L and BL, and L and BH from 2nd h onwards up to 24 h postoperatively. Similar reduced VAS pain scores were also reported by Paliwal and Karnawat (2013).
In group BH patients in the 1st 1 h after LPB administration, a modified RSS of 1.93 ± 0.86 was achieved. This was statistically significant from the control group (p = 0.003) and between the groups BL (p = 0.043). This increase in sedation in the group BH led to respiratory depression in these patients, causing a decrease in the arterial oxygen saturation. These patients required active awakening and supplemental oxygen to prevent any hypoxemia associated adverse events. There was no residual sedation in postoperative period after 2 h of performing block in either of the 3 groups.
In the study by Paliwal and Karnawat (2013), a similar sedation was encountered in 40% of the patients in the 300 μg buprenorphine group, as compared to none in the control group. However, the effects of a dose of 150 μg were not studied. So, a difference between the sedation effects of different doses of buprenorphine could not be commented upon. They had also not compared the sedation scores statistically. We could not find any other studies comparing the sedation scores in the postoperative period. Therefore, more studies are needed to evaluate this parameter.
Further limitations of the current study include non-inclusion of the elderly age group which would be more vulnerable to hemodynamic variations and respiratory depression than the ASA 1and 2 participants enrolled in the present study.
Conclusion
Buprenorphine in doses of 150 and 300 μg as an adjuvant to levobupivacaine in lumbar plexus block reduces postoperative pain scores, thus prolonging the duration of analgesia and decreasing the requirement of analgesics. However, a dose of 300 μg may lead to significant adverse events such as sedation and respiratory depression. Hence, buprenorphine in a dose of 150 μg as an adjuvant to levobupivacaine in lumbar plexus block is more suitable for postoperative analgesia.
Availability of data and materials
This shall be made available on request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- Dr.:
-
Doctor
- R.P.G.M.C:
-
Rajendra Prasad Government Medical College
- inj.:
-
Injection
- LA:
-
Local anesthetic
- LPB:
-
Lumbar plexus block
- RSS:
-
Ramsay Sedation Score
- SAB:
-
Subarachnoid block
- SD:
-
Standard deviation
- SpO2 :
-
Oxygen saturation
- SPSS:
-
Statistical Package for Social Sciences
- VAS:
-
Visual analog scale
- PACU:
-
Post-anesthesia care unit
- MS Excel:
-
Microsoft Excel
- USG:
-
Ultrasound
- no.:
-
Number
- NOP:
-
Nociceptin opioid receptor
- ORL1:
-
Opioid receptor like-1
References
Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W (1998) A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 46:245–249
Behr A, Freo U, Ori C, Westermann B, Alemanno F (2012) Buprenorphine added to levobupivacaine enhances postoperative analgesia of middle interscalene brachial plexus block. J Anesth 26:746–751
Candido KD, Winnie AP, Ghaleb AH, Fattouh MW, Franco CD (2002) Buprenorphine added to the local anesthetic for axillary brachial plexus block prolongs postoperative analgesia. Reg Anesth Pain Med 27:162–167
Connor M, Vaughan CW, Chieng B, Christie MJ (1996b) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurons in vitro. Br J Pharmacol 119:1614–1618
Connor M, Yeo A, Henderson G (1996a) The effect of nociception on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol 118:205–207
Fukuda K (2015) Opioid analgesics. In: Miller RD, Cohen NH, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL (eds) Miller’s anesthesia, 8th edn. Saunders Elsevier, Philadelphia, pp 864–814
Gonzalez-Suarez S, Pacheco M, Roige J, Puig MM (2009) Comparative study of ropivacaine 0.5% and levobupivacaine 0.33% in axillary brachial plexus block. Reg Anesth Pain Med 34:414–419
Gormley WP, Murray JM, Fee JPH, Bower S (1996) Effect of the addition of alfentanil to lignocaine during axillary brachial plexus anaesthesia. Br J Anaesth 76:802–805
Jadon A, Panigrahi MR, Parida SS, Chakraborty S, Agrawal PS, Panda A (2009) Buprenorphine improves the efficacy of bupivacaine in nerve plexus block: a double blind randomized evaluation in subclavian perivascular brachial block. J Anaesth Clin Pharmacol 25:207–210
Jain N, Khare A, Khandelwal S, Mathur P, Singh M, Mathur V (2017) Buprenorphine as an adjuvant to 0.5% ropivacaine for ultrasound-guided supraclavicular brachial plexus block: a randomized, double-blind, prospective study. Ind J Pain 31:112–118
Knoflach F, Reinscheid RK, Civelli O, Kemp JA (1996) Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci 16:6657–6664
Laduron PM (1984) Axonal transport of opiate receptors in capsaicin-sensitive neurons. Brain Res 294:157–160
Leffler A, Frank G, Kistner K, Niedermirtl F, Koppert W, Reeh PW et al (2012) Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial mu-opioid receptor agonist buprenorphine. Anesthesiology 116:1335–1346
Hadzic A, Carrera A, Clark TB, Gadsden J, Karmakar MK, Sala-Blanch X et al (2012) Lumbar plexus block. Hadzic’s peripheral nerve blocks and anatomy for ultrasound-guided regional anesthesia, 2nd edn. McGraw Hill, New Delhi, pp 217–228.
Lutfy K, Cowan A (2004) Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2:395–302
Meunier JC (1997) Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol 340:1–15
Murdoch JA, Dickson UK, Wilson PA, Berman JS, Gad-Elrab RR, Scott NB (2002) The efficacy and safety of three concentrations of levobupivacaine administered as a continuous epidural infusion in patients undergoing orthopedic surgery. Anesth Analg 94(2):438–444
Paliwal B, Karnawat R (2013) Comparative study of effects of buprenorphine or clonidine as adjuvants to local anesthetics (bupivacaine 0.25%) for supraclavicular brachial plexus block. IOSR J Dent Med Sci 4:30–39
Panditrao MM, Mathew BI, Panditrao MM (2011) Comparison of two different strengths of buprenorphine hydrochloride added to local anaesthetics “pre-emptively” in supraclavicular brachial plexus block: does it act centrally or peripherally? Indian J Pain 25:32–38
Sauter AR, Ullensvang K, Niemi G, Lorentzen HT, Bendtsen TF, Børglum J et al (2015) The Shamrock lumbar plexus block: a dose-finding study. Eur J Anaesthesiol 32:764–770
Sherif A, Nabil AEM, Azza Y, Raouf R, Raham H (2011) Lumbar plexus block as a method of postoperative analgesia after hip surgery. Egypt J Anaesth 27:127–133
Stevens RD, Van Gessel E, Flory N, Fournier R, Gamulin Z (2000) Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology 93:115–121
Takahashi T, Okubo K, Kojima S, Nishikawa H, Takemura M, Tsubota-Matsunami M et al (2013) Antihyperalgesic effect of buprenorphine involves nociception/orphanin FQ peptide-receptor activation in rats with spinal nerve injury-induced neuropathy. J Pharmacol Sci 122:51–54
Vaughan CW, Christie MJ (1996) Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociception, of inwardly rectifying K conductance in dorsal raphe nucleus neurons. Br J Pharmacol 117:1609–1611
Wajima Z, Nakajima Y, Kim C, Kobayashi N, Kadotani H, Adachi H et al (1995) IV compared with brachial plexus infusion of butorphanol for postoperative analgesia. Br J Anaesth 74:392–395
Acknowledgements
We thank Dr. Shelly Rana, Professor, Department of Anesthesia, Dr Rajendra Prasad Government Medical College Kangra at Tanda, Himachal Pradesh, India, for her guidance and technical support.
Funding
The authors declare that no funding was involved.
Author information
Authors and Affiliations
Contributions
VT, JS, and LT significantly contributed in the conception and design of work. VT and AM contributed to the literature search, acquisition, analysis, and interpretation of data. VT and JS contributed in the manuscript preparation editing and review. The corresponding author participated in all the phases. All the authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Institutional Ethics Committee (IEC) Registration number ERC/490/Inst/HP/2013 of Dr Rajendra Prasad Government Medical College Kangra at Tanda, Himachal Pradesh, India. Protocol no 60/2016. The procedure of lumbar plexus block was explained to the patient to obtain written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tulsyan, V., Singh, J., Thakur, L. et al. A comparative study of buprenorphine in two different doses as an adjuvant to levobupivacaine in US-guided lumbar plexus block for postoperative analgesia. Ain-Shams J Anesthesiol 13, 7 (2021). https://doi.org/10.1186/s42077-021-00126-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-021-00126-w