Abstract
Purpose
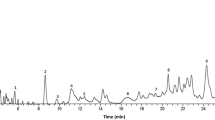
This study investigated the protective and antioxidative effect of aqueous root extract of ginger (Zingiber officinale) (AREG) against lead (Pb)-induced neurotoxicity in adult Wistar rats.
Methods
Twenty (20) adult Wistar rats were randomly divided into four groups (A–D) of five animals each: Group A served as the control group, Group B received 100 mg/kg body weight of Pb only, Group C received 100 mg/kg body weight of AREG pre-treatment 1 h before administration of 100 mg/kg body weight of Pb, and Group D received 100 mg/kg body weight of AREG only. At the end of the experiment, neurobehavioral activity, lipid peroxidation, antioxidant enzymes activity, and histology of the cerebrum and cerebellum were evaluated.
Results
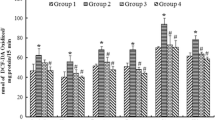
Rats treated with Pb alone had a significant reduction in final body weight, absolute brain weight, SOD, and CAT activity when compared to control. Similarly, there was a significant reduction in ambulation and rearing episodes as well as a significant increase in immobility and self-grooming activities in rats treated with Pb alone, thus indicating impaired locomotor functions. A significant elevation in lipid peroxidation was also observed. However, pretreatment of rats with AREG attenuated the effects induced by Pb.
Conclusion
Based on the results, AREG is a promising neuroprotective agent for preventing Pb-induced impairment of locomotor functions and dysregulation of antioxidant enzymes activity. It could be a viable alternative to other synthetic medicines utilized in the attenuation of Pb-induced toxicity.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
El-Tantawy W. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med. 2015;2015(6):327–31.
Shaffer RM, Gilbert SG. Reducing occupational lead exposures: strengthened standards for a healthy workforce. Neurotoxicology. 2018;69:181–6.
Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115(3):463–71.
Chowdhury R, Sarnat SE, Darrow L, McClellan W, Steenland K. Mortality among participants in a lead surveillance program. Environ Res. 2014;132:100–4.
Liu J, Han D, Li Y, Zheng L, Gu C, Piao Z, et al. Lead affects apoptosis and related gene XIAP and Smac expression in the hippocampus of developing rats. Neurochem Res. 2010;35(3):473–9.
Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58.
Pande M, Flora S. Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology. 2002;177(2–3):187–96.
Ghareeb DA, Hussien HM, Khalil AA, El-Saadani MA, Ali AN. Toxic effects of lead exposure on the brain of rats: Involvement of oxidative stress, inflammation, acetylcholinesterase, and the beneficial role of flaxseed extract. Toxicol Environ Chem. 2010; 92(1):187–95.
Kabeer A, Mailafiya M, Danmaigoro A, Rahim E, Bakar Z. Therapeutic potential of curcumin against lead-induced toxicity: a review. Biomed Res Ther. 2019;6:3053–66.
Choi JG, Kim SY, Jeong M, Oh MS. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol Ther. 2018;182:56–69.
Reddy YA, Chalamaiah M, Ramesh B, Balaji G, Indira P (2014) Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sci Technol 51(5):908–14
Wang W, Wang Z. Studies of commonly used traditional medicine-ginger Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese materia medica. 2005;30(20):1569–73.
Prasad S, Tyagi AK. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer Gastroenterology research and practice 2015. 2015;.
Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11(2).
Khaki A, FATHI AF, Nouri M, Khaki AA, Ozanci CC, Ghafari NM, et al. The effects of Ginger on spermatogenesis and sperm parameters of rat. 2009.
Sofowora A. Phytochemical screening of medicinal plants and traditional medicine in Africa. Spectrum Books Limited Nigeria. 1993; pp150–156.
Trease G, Evans W. Text book of Pharmacognosy London Bailliare. Tindall. 1983;12(193):336.
Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54(4):275–87.
Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83–244.
Cauli O, Morelli M. Subchronic caffeine administration sensitizes rats to the motor-activating effects of dopamine D 1 and D 2 receptor agonists. Psychopharmacology (Berl). 2002;162(3):246–54.
Kim H-J, Kong M-K, Kim Y-C. Beneficial effects of Phellodendri Cortex extract on hyperglycemia and diabetic nephropathy in streptozotocin-induced diabetic rats. Bmb Rep. 2008;41(10):710–5.
Montilla P, Barcos M, Munoz MC, Bujalance I, Munoz-Castaneda JR, Tunez I. Red wine prevents brain oxidative stress and nephropathy in streptozotocin-induced diabetic rats. J Biochem Mol Biol. 2005;38(5):539.
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500.
Aebi H. Catalase in vitro Methods. Enzymol. 1984;105:121–6.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Drury R, Wallington E. Carleton’s histological technique 5th ed New York: Churchill Livingstone. 1980.
Amjad Z, Iqbal MZ, Shoro AA. Lead-induced reduction in body and kidney weight of Wistar albino rats ameliorated by Ginkgo biloba extract (EGb 761) Biochem Physiol. 2013;2(113):2.
Rafique M, Perveen K, Khan N, Nigar S. Lead intoxication causing loss of body weight and loss of absolute weight of testes in albino rats. Hamdard Med. 2008;51:123–8.
Klaassen CD. Heavy metals and heavy-metal antagonists The pharmacological basis of therapeutics. 1996;1649–71.
Canli M, Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut. 2003;121(1):129–36.
Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15(8):1753–65.
Kondo H, Nakagaki I, Sasaki S, Hori S, Itokawa Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization American Journal of Physiology-Endocrinology And. Metabolism. 1993;265(6):E839–44.
Hall CS. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18(3):385.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33.
Gould TD, Dao DT, Kovacsics CE. The open field test. Mood and anxiety related phenotypes in mice: Springer; 2009. p. 1–20.
Erdoğan F, Gölgeli A, Arman F, Ersoy AÖ (2004) The effects of pentylenetetrazole-induced status epilepticus on behavior, emotional memory, and learning in rats Epilepsy Behav 5(3):388–93.
Ennaceur A. Tests of unconditioned anxiety—pitfalls and disappointments. Physiol Behav. 2014;135:55–71.
Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46(3):321–40.
Costall B, Jones B, Kelly M, Naylor RJ, Tomkins D. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32(3):777–85.
Masood A, Banerjee B, Vijayan V, Ray A. Modulation of stress-induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol. 2003;458(1–2):135–9.
Saikhedkar N, Bhatnagar M, Jain A, Sukhwal P, Sharma C, Jaiswal N. Effects of mobile phone radiation (900 MHz radiofrequency) on structure and functions of rat brain. Neurol Res. 2014;36(12):1072–9.
Ranjbar H, Radahmadi M, Reisi P, Alaei H. Effects of electrical lesion of basolateral amygdala nucleus on rat anxiety‐like behaviour under acute, sub‐chronic, and chronic stresses. Clin Exp Pharmacol Physiol. 2017;44(4):470–9.
Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2(10):2538.
Kalueff AV, Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur J Pharmacol. 2005;508(1–3):147–53.
Attia A, Ibrahim F, Nabil GM, Aziz SW. Antioxidant effects of ginger (Zingiber officinale Roscoe) against lead acetate-induced hepatotoxicity in rats. Afr J Pharm Pharmacol. 2013;7(20):1213–9.
Sudjarwo SA, Giftania Wardani Sudjarwo K. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res Pharm Sci. 2017;12(5):381.
Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev 2012. 2012
Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154–66.
Soltaninejad K, Kebriaeezadeh A, Minaiee B, Ostad SN, Hosseini R, Azizi E, et al. Biochemical and ultrastructural evidences for toxicity of lead through free radicals in rat brain. Hum Exp Toxicol. 2003;22(8):417–23.
Paulis MG, Hassan OA, Abbass MF, Mohammad MA-AH. Structural and lipid peroxidation effects of lead on rat hippocampus and its attenuation by hydrogen rich water. J Chem Neuroanat. 2018;91:55–62.
Singh PK, Singh MK, Yadav RS, Dixit RK, Mehrotra A, Nath R. Attenuation of lead-induced neurotoxicity by omega-3 fatty acid in rats. Ann Neurosci. 2017;24(4):221–32.
Clasen RA, Hartmann JF, Coogan PS, Pandolfi S, Laing I, Becker RA. Experimental acute lead encephalopathy in the juvenile rhesus monkey. Environ Health Perspect. 1974;7:175–85.
Fonumm F, Lock E. Cerebellum as a target for toxic substance. Toxicol Lett. 2000;15(112–113):9–16.
Enogieru AB, Momodu OI. The developing cerebellum as a target for toxic substances: protective role of antioxidants. The Cerebellum. 2021;:1–17.
Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995;15(3):2040–56.
Fukuda K, Aihara N, Sagar SM, Sharp FR, Pitts LH, Honkaniemi J, et al. Purkinje cell vulnerability to mild traumatic brain injury. J Neurotrauma. 1996;13(5):255–66.
Abubakar K, Muhammad Mailafiya M, Danmaigoro A, Musa Chiroma S, Abdul Rahim EB. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules. 2019;9(9):453.
Lazarus SS, Adebisi SS, Tanko Y, Agbon AN, Budaye MN. Histological and histochemical assessements on the effect of ethanol fruit extract of Phoenix dactylifera L.(Date Palm) on cerebral cortex of lead acetate treated wistar rats. Afr J Cell Pathol. 2018;10(1):1–9.
Feroz S, Mohamad S, Lee G, Malek S, Tayyab S. Supramolecular interaction of 6-shogaol, a therapeutic agent of Zingiber officinale with human serum albumin as elucidated by spectroscopic, calorimetric and molecular docking methods. Phytomedicine. 2015;22(6):621–30.
Manasa D, Srinivas P, Sowbhagya H. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013;139(1–4):509–14.
Acknowledgements
The authors appreciate the kind assistance of Mr. Kevin Akonofua during the conduct of this research.
Author information
Authors and Affiliations
Contributions
ABE conceived, designed, and performed the experiments; ABE and OM performed the analysis, interpreted the data, and prepared the draft of the manuscript. All authors have reviewed and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The experimental procedures performed on the animals were according to the Research Ethics Committee of the College of Medical Sciences, University of Benin, Nigeria, with approval number CMS|REC|2021|158. The use of rats for the study was also according to the Ethical Guidelines Involving Whole Animal Testing of the Research Ethics Committee of the College of Medical Sciences, University of Benin, Nigeria.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enogieru, A.B., Momodu, O. Neuroprotective effects of Zingiber officinale against lead-induced toxicity in Wistar rats. Nutrire 48, 2 (2023). https://doi.org/10.1186/s41110-022-00184-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-022-00184-6