Abstract
Cholangiocarcinoma (CHOL) is one of the most aggressive tumors worldwide and cannot be effectively treated by conventional and novel treatments, including immune checkpoint blockade therapy. The mRNA vaccine-based immunotherapeutic strategy has attracted much attention for various diseases, however, its application in CHOL is limited due to the thoughtlessness in the integration of vaccine design and patient selection. A recent study established an integrated path for identifying potent CHOL antigens for mRNA vaccine development and a precise stratification for identifying CHOL patients who can benefit from the mRNA vaccines. In spite of a promising prospect, further investigations should identify immunogenic antigens and onco-immunological characteristics of CHOL to guide the clinical application of CHOL mRNA vaccines in the future.
Similar content being viewed by others
Background
Cholangiocarcinoma (CHOL) is the second most common hepatobiliary cancer and one of the most aggressive tumors worldwide [1]. Surgical resection can be curative for some CHOL patients who present with early-stage disease [1, 2]. However, CHOL is mostly detected at an advanced stage, and thus most patients are late for surgery [3]. Besides, most patients with unresectable CHOL get little benefit from systematic treatments. For instance, gemcitabine and cisplatin, as first-line treatments for CHOL, can only achieve an overall survival of less than 1 year [4]. Although fibroblast growth factor receptor and isocitrate dehydrogenase mutations-targeted drugs, as second-line treatments, can achieve a 30–35% response rate, most CHOL patients do not have such mutations and are thus not suitable for such targeted therapies [5,6,7]. Additionally, mitogen-activated protein kinase inhibitor selumetinib shows poor efficacy in patients with metastatic biliary cancer [8], while hepatocyte growth factor receptor inhibitor tivantinib combined with gemcitabine has a partial response in only 20% of patients [9].
Current dilemma in CHOL immunotherapy
Since immune system plays a critical role in recognition and elimination of malignant cells, intensive studies have been conducted to seek effective approaches for activating anti-tumor immune response in the past 2 decades [10]. The immune checkpoint proteins, including programmed death (ligand) 1 [PD-(L)1] and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), help tumor cells escape from immune surveillance, and thus immune checkpoint blockade (ICB) therapy targeting such proteins has been approved to treat a wide range of tumors [10,11,12]. Although ICB has achieved great success in cancer immunotherapy in recent years, it has limited efficacy in CHOL [13, 14]. In KEYNOTE028, 24 CHOL patients were enrolled in a basket trial involving PD-L1 positive solid tumor and treated with anti-PD-1 antibody pembrolizumab, of which only 4 patients (17%) achieved a partial response. Furthermore, the response rate to ICB was even lower in non-mis-match repair (MMR) deficient CHOL, although the data were mixed [15]. In KEYNOTE-158, a response rate of only 5.8% was detected among 104 patients without known MMR deficiency, with a progression-free survival of less than 2 months [7, 15]. Although the response rate of patients with MMR deficiency to ICB exceeded 40%, such MMR deficiency cases only occur in 5% of CHOL patients [3, 7]. Previous studies have demonstrated that tumor response to immunotherapy largely depends on the expression levels of tumor-specific antigens that mediate immune recognition on tumors and promote activation of anti-tumor immunity [16,17,18]. Tumor cells escape immune surveillance by reducing the expression of immunogenic antigens or modifying certain antigens through multiple mechanisms, while lack of tumor-specific antigen stimulation can lead to T-cell exhaustion, eventually resulting in primary and acquired tumor resistance to immunotherapy [19,20,21,22,23].
Tumor vaccines for CHOL treatment
Tumor vaccines activating anti-tumor immunity with immunogenic tumor-specific or nonspecific antigens are emerging therapeutic strategies for CHOL [23, 24]. Several vaccine-based strategies, including carcinoembryonic antigen RNA-pulsed dendritic cell (DC) and immunogenic peptides plus gemcitabine, have been developed for CHOL treatment, singly or in combination (Table 1) [25,26,27]. The mRNA vaccine has attracted much attention in the past decades since it can stimulate immune activation through multiple mechanisms [28]. Conventionally, mRNA vaccines can be processed as normal endogenous mRNAs in antigen-presenting cells (APCs), inducing antigen expression and presentation on major histocompatibility complex class I (MHC-I) for CD8+ T cell activation and major histocompatibility complex class II (MHC-II) for CD4+ T cell activation [14, 24]. Moreover, mRNA can activate immune systems via Toll-like receptors (TLR3, TLR7, and TLR8) located on the plasma membrane or endosome of APCs, leading to cytokine production and providing an essential costimulatory signal for the activation of T and B cells [29, 30]. Additionally, mRNA can upregulate chemokine production in non-immune cells through sensing by RIG-I-like receptors, thus recruiting APCs and other immune cells to the injection sites [31, 32]. Notably, mRNA vaccines have more advantages than previously studied vaccine models for CHOL. First, mRNA vaccines can be produced on a large scale within a short period due to their mature manufacturing and cost-effective process [24, 33]. Second, mRNA vaccines can mediate the transient expression of the selected antigens without the risk of being integrated into the host genome and thus are much safer than DNA and viral vaccines [24, 34]. Third, mRNA vaccines can relatively alleviate adverse effects due to the short-term expression of encoded peptides [33, 34]. Finally, the mRNA-based techniques allow the development of a personalized treatment based on individual sequencing data of tumor samples [24, 33]. As a result, the mRNA vaccines may become the next hotspot in cancer immunotherapy (Table 2) [35,36,37,38,39,40,41,42].
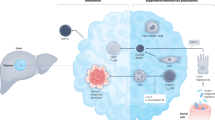
An integrated path to identify potent CHOL antigens for mRNA vaccine development
The precise identification of most immunogenic stimulators as potent candidates for optimal immune response and therapeutic efficacy is one of the critical steps for mRNA vaccine development. Previous studies have demonstrated that bioinformatics can help identify therapeutic targets and antigens and help understand tumor-host interplay from an immune perspective. cBioPortal for Cancer Genomics, Gene Expression Profiling Interactive Analysis (GEPIA), and Tumor Immune Estimation Resource (TIMER) are well-established, easily accessible databases that largely lower the threshold of bioinformatics analysis. Particularly, the cBioPortal for Cancer Genomics is an online tool used to analyze the raw data from large-scale genomic projects, including The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC) [43]. GEPIA is an online resource integrating RNA sequencing data from both tumors and normal samples of the TCGA and the Genotype-Tissue Expression (GTEx) programs [44]. TIMER is an online tool for immune infiltration analysis across discrete cancers [45]. Based on these online databases and bioinformatic approaches, a new pipeline has been established to identify promising antigens for mRNA CHOL vaccine development [46]. Briefly, 1391 genes with both amplified copy numbers and mutations in CHOL were first determined as potential candidates. Subsequently, the prognostic values of the amplified and mutated genes were analyzed. Fifteen candidates were identified as closely associated with overall survival, of which 3 were also correlated with progression-free survival. Elevated mRNA levels of the three candidates [transformation/transcription domain associated protein (TRRAP), fragment crystallizable (Fc) fragment of IgG receptor 1A (FCGR1A), and CD247] were significantly correlated with a better prognosis of CHOL. Moreover, the elevated expression levels of FCGR1A, TRRAP, and CD247 were closely associated with the infiltration of DCs, macrophages, and B cells. As a result, the three antigens were identified as potential candidates for the development of CHOL mRNA vaccine, boosting anti-tumor immunity after delivery to the adapted immune system.
A precise stratification for the identification of suitable CHOL patients for mRNA vaccine
Identifying suitable patients with the highest probability of benefiting from the treatment is also crucial for the mRNA vaccine development process [47]. Mounting evidence indicates that the distinction of tumor immune microenvironment largely determines the immunotherapeutic efficacy in different cancer patients [22]. As a result, the CHOL patients have recently been stratified into two distinct immune subtypes (ISs) with the maximum intergroup and the minimum intragroup variances (IS1 and IS2, respectively) based on the gene profiles extracted from TCGA and Gene Expression Omnibus (GEO) [46]. ISs are closely correlated with the prognosis of CHOL patients. Moreover, genes associated with immunogenic cell death and immune checkpoint are differentially expressed in the two ISs. The immune cell components in these two ISs have also been analyzed. IS1 has an immune “hot” phenotype with increased scores of activated CD8 T cells, memory T cells, and immune-suppressive cell subsets [Myeloid-derived suppressor cell (MDSC), Macrophage, and Treg]. In contrast, IS2 has an immune “cold” phenotype with decreased immune cell infiltration. The molecular signature analysis has further confirmed the immune characteristics of the two ISs, indicating that IS2 patients may benefit from mRNA vaccine for immune status alteration. The intergroup heterogeneity of ISs has been visualized through the construction of the immune landscape of CHOL, where the two ISs were further divided into smaller subgroups with distinct prognoses, thus providing valuable information for patient selection for mRNA vaccine treatment. Finally, the immune gene co-expression modules have been analyzed to identify immune hub for selecting suitable CHOL patients for treatment with mRNA vaccines [22].
Conclusions
The novel pipeline collectively provides a practical approach for the development of an mRNA vaccine for CHOL, particularly in selecting potent antigens and appropriate patients. Notably, although treatment with mRNA vaccines is promising, mRNA vaccines for CHOL patients are still in the initial stages and may face several challenges in the future. First, an in silico study has identified potential CHOL antigens based on integrated information of expression, mutation, prognostic value, and correlation with infiltrated immune cells, however, it is difficult to estimate whether such candidates can induce anti-CHOL immunity in vivo. Moreover, at advanced stages, CHOL may have already developed numerous mechanisms for immune escape, eventually causing therapeutic resistance. Additionally, the requirement for activation of anti-tumor immunity is highly variable among different patients due to CHOL heterogeneity. Last but not least, the required dosage of mRNA vaccine to treat tumors is usually higher than prophylactic vaccination for infectious diseases, which may raise concerns about the safety of mRNA vaccines in CHOL patients. Therefore, advanced investigations focusing on understanding the onco-immunological characteristics of CHOL, especially the intra-tumoral heterogeneity, tumor-microenvironmental crosstalk, and microenvironmental instability, are needed for precise and feasible application of CHOL mRNA vaccine in clinics, improvement of its effectiveness and safety, as well as the prognosis of CHOL patients.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- APC:
-
Antigen-presenting cell
- CHOL:
-
Cholangiocarcinoma
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DC:
-
Dendritic cell
- GEO:
-
Gene expression omnibus
- GEPIA:
-
Gene expression profiling interactive analysis
- GTEx:
-
Genotype-tissue expression
- ICB:
-
Immune checkpoint blockade
- ICGC:
-
International cancer genome consortium
- IS:
-
Immune subtype
- MDSC:
-
Myeloid-derived suppressor cell
- MHC-I:
-
Major histocompatibility complex class I
- MHC-II:
-
Major histocompatibility complex class II
- MMR:
-
Mis-match repair
- PD-(L)1:
-
Programmed death (ligand)-1
- TCGA:
-
The cancer genome atlas
- TLR:
-
Toll-like receptor
- TIMER:
-
Tumor immune estimation resource
References
Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7(1):65.
Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–44.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353–63.
Bridgewater J, Lopes A, Beare S, Duggan M, Lee D, Ricamara M, et al. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer. 2016;16:153.
Pant S, Saleh M, Bendell J, Infante JR, Jones S, Kurkjian CD, et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol. 2014;25(7):1416–21.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82.
Huang X, Tang T, Zhang G, Hong Z, Xu J, Yadav DK, et al. Genomic investigation of co-targeting tumor immune microenvironment and immune checkpoints in pan-cancer immunotherapy. NPJ Precis Oncol. 2020;4(1):29.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6(9):1405–9.
Manne A, Woods E, Tsung A, Mittra A. Biliary tract cancers: treatment updates and future directions in the era of precision medicine and immuno-oncology. Front Oncol. 2021;11:768009.
Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147(8):2190–8.
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12(1):93.
Chen H, Yang M, Wang Q, Song F, Li X, Chen K. The new identified biomarkers determine sensitivity to immune check-point blockade therapies in melanoma. Oncoimmunology. 2019;8(8):1608132.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74.
Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017;17(1):38–53.
Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):72.
Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov. 2021;20(12):899–919.
Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–29.
Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms' tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20(16):4228–39.
Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19(2):171–8.
Löfffler MW, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M, et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016;65(4):849–55.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8.
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9.
Kowalczyk A, Doener F, Zanzinger K, Noth J, Baumhof P, Fotin-Mleczek M, et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34(33):3882–93.
Edwards DK, Jasny E, Yoon H, Horscroft N, Schanen B, Geter T, et al. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J Transl Med. 2017;15(1):1.
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–60.
Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer. 2021;20(1):52.
Van Tendeloo VF, van de Velde A, van Driessche A, Cools N, Anguille S, Ladell K, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107(31):13824–9.
Lesterhuis WJ, de Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30(12):5091–7.
Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. J Immunother Cancer. 2015;3:14.
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–12.
Kyte JA, Aamdal S, Dueland S, Saeboe-Larsen S, Inderberg EM, Madsbu UE, et al. Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells. Oncoimmunology. 2016;5(11):e1232237.
Bol KF, Figdor CG, Aarntzen EH, Welzen ME, van Rossum MM, Blokx WA, et al. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. Oncoimmunology. 2015;4(8):e1019197.
Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507.
Khoury HJ, Collins RH, Blum W, Stiff PS, Elias L, Lebkowski JS, et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer. 2017;123(16):3061–72.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):l1.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98-102.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–10.
Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer. 2021;20:50.
Roudko V, Greenbaum B, Bhardwaj N. Computational prediction and validation of tumor-associated neoantigens. Front Immunol. 2020;11:27.
Acknowledgements
The author XH would like to express his deepest gratitude to Prof. Guido Kroemer for cancer immunity-associated technological training, conceptual inspiration, and moral edification in his laboratory.
Funding
This work was supported by the National Natural Science Foundation of China (31970696, 81502975, 81830089, U20A20378, 82188102), Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholar (LR22H160010), National Key Research and Development Program (2019YFC1316000), Zhejiang Provincial Key Research and Development Program (2019C03019), and Zhejiang Provincial College Student Science and Technology Innovation Activity Plan-College Student Innovation and Entrepreneurship Incubation Program (Young Talent Program) (2022R40122).
Author information
Authors and Affiliations
Contributions
XH and TBL conceived the manuscript and share the senior authorship. TYT and XH contributed equally to the literature review and writing. GZ and MHL helped in proof-reading. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, TY., Huang, X., Zhang, G. et al. mRNA vaccine development for cholangiocarcinoma: a precise pipeline. Military Med Res 9, 40 (2022). https://doi.org/10.1186/s40779-022-00399-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40779-022-00399-8